This is a preprint.
Cytoskeletal adaptation following long-term dysregulation of actomyosin in neuronal processes
- PMID: 37662186
- PMCID: PMC10473725
- DOI: 10.1101/2023.08.25.554891
Cytoskeletal adaptation following long-term dysregulation of actomyosin in neuronal processes
Update in
-
Prolonged depletion of profilin 1 or F-actin causes an adaptive response in microtubules.J Cell Biol. 2024 Jul 1;223(7):e202309097. doi: 10.1083/jcb.202309097. Epub 2024 May 9. J Cell Biol. 2024. PMID: 38722279 Free PMC article.
Abstract
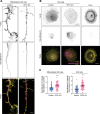
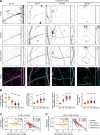
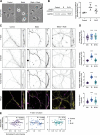
Microtubules, intermediate filaments, and actin are cytoskeletal polymer networks found within the cell. While each has unique functions, all the cytoskeletal elements must work together for cellular mechanics to be fully operative. This is achieved through crosstalk mechanisms whereby the different networks influence each other through signaling pathways and direct interactions. Because crosstalk can be complex, it is possible for perturbations in one cytoskeletal element to affect the others in ways that are difficult to predict. Here we investigated how long-term changes to the actin cytoskeleton affect microtubules and intermediate filaments. Reducing F-actin or actomyosin contractility increased acetylated microtubules and intermediate filament expression, with the effect being significantly more pronounced in neuronal processes. Changes to microtubules were completely reversible if F-actin and myosin activity is restored. Moreover, the altered microtubules in neuronal processes resulting from F-actin depletion caused significant changes to microtubule-based transport, mimicking phenotypes that are linked to neurodegenerative disease. Thus, defects in actin dynamics cause a compensatory response in other cytoskeleton components which profoundly alters cellular function.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
