This is a preprint.
Disturbance-Diversity Relationships of Microbial Communities Change Based on Growth Substrate
- PMID: 37662195
- PMCID: PMC10473689
- DOI: 10.1101/2023.08.25.554838
Disturbance-Diversity Relationships of Microbial Communities Change Based on Growth Substrate
Update in
-
Disturbance-diversity relationships of microbial communities change based on growth substrate.mSystems. 2024 Feb 20;9(2):e0088723. doi: 10.1128/msystems.00887-23. Epub 2024 Jan 23. mSystems. 2024. PMID: 38259105 Free PMC article.
Abstract
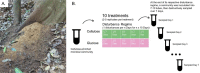
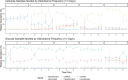
Disturbance events can impact ecological community dynamics. Understanding how communities respond to disturbances, and how those responses can vary, is a challenge in microbial ecology. In this study, we grew a previously enriched specialized microbial community on either cellulose or glucose as a sole carbon source, and subjected them to one of five different disturbance regimes of varying frequencies ranging from low to high. Using 16S rRNA gene amplicon sequencing, we show that community structure is largely driven by substrate, but disturbance frequency affects community composition and successional dynamics. When grown on cellulose, bacteria in the genera Cellvibrio, Lacunisphaera, and Asticaccacaulis are the most abundant microbes. However, Lacunisphaera is only abundant in the lower disturbance frequency treatments, while Asticaccaulis is more abundant in the highest disturbance frequency treatment. When grown on glucose, the most abundant microbes are two Pseudomonas sequence variants, and a Cohnella sequence variant that is only abundant in the highest disturbance frequency treatment. Communities grown on cellulose exhibited a greater range of diversity (0.67-1.99 Shannon diversity and 1.38-5.25 Inverse Simpson diversity) that peak at the intermediate disturbance frequency treatment, or 1 disturbance every 3 days. Communities grown on glucose, however, ranged from 0.49-1.43 Shannon diversity and 1.37- 3.52 Inverse Simpson with peak diversity at the greatest disturbance frequency treatment. These results demonstrate that the dynamics of a microbial community can vary depending on substrate and the disturbance frequency, and may potentially explain the variety of diversity-disturbance relationships observed in microbial ecosystems.
Figures


References
-
- Plante C. J. Defining Disturbance for Microbial Ecology. Microb. Ecol. 74, 259–263 (2017). - PubMed
-
- Sousa W. P. The Role of Disturbance in Natural Communities. www.annualreviews.org (1984).
-
- Rykiel E. J. Towards a definition of ecological disturbance. Austral Ecol. 10, 361–365 (1985).
-
- Connell J. H. Diversity in Tropical Rain Forests and Coral Reefs. New Series vol. 199 1302–1310 (1978). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
