This is a preprint.
Niche-specific macrophage loss promotes skin capillary aging
- PMID: 37662387
- PMCID: PMC10473701
- DOI: 10.1101/2023.08.25.554832
Niche-specific macrophage loss promotes skin capillary aging
Update in
-
Niche-specific dermal macrophage loss promotes skin capillary ageing.Nature. 2025 Oct 15. doi: 10.1038/s41586-025-09639-y. Online ahead of print. Nature. 2025. PMID: 41094141
Abstract
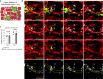
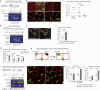
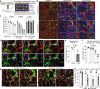
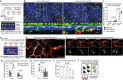
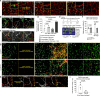
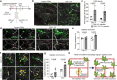
All mammalian organs depend upon resident macrophage populations to coordinate repair processes and facilitate tissue-specific functions1-3. Recent work has established that functionally distinct macrophage populations reside in discrete tissue niches and are replenished through some combination of local proliferation and monocyte recruitment4,5. Moreover, decline in macrophage abundance and function in tissues has been shown to contribute to many age-associated pathologies, such as atherosclerosis, cancer, and neurodegeneration6-8. Despite these advances, the cellular mechanisms that coordinate macrophage organization and replenishment within an aging tissue niche remain largely unknown. Here we show that capillary-associated macrophages (CAMs) are selectively lost over time, which contributes to impaired vascular repair and tissue perfusion in older mice. To investigate resident macrophage behavior in vivo, we have employed intravital two-photon microscopy to non-invasively image in live mice the skin capillary plexus, a spatially well-defined model of niche aging that undergoes rarefication and functional decline with age. We find that CAMs are lost with age at a rate that outpaces that of capillary loss, leading to the progressive accumulation of capillary niches without an associated macrophage in both mice and humans. Phagocytic activity of CAMs was locally required to repair obstructed capillary blood flow, leaving macrophage-less niches selectively vulnerable to both homeostatic and injury-induced loss in blood flow. Our work demonstrates that homeostatic renewal of resident macrophages is not as finely tuned as has been previously suggested9-11. Specifically, we found that neighboring macrophages do not proliferate or reorganize sufficiently to maintain an optimal population across the skin capillary niche in the absence of additional cues from acute tissue damage or increased abundance of growth factors, such as colony stimulating factor 1 (CSF1). Such limitations in homeostatic renewal and organization of various niche-resident cell types are potentially early contributors to tissue aging, which may provide novel opportunities for future therapeutic interventions.
Keywords: Aging; Blood Capillaries; Intravital Microscopy; Resident Macrophages; Tissue Repair.
Figures






References
-
- Nobs S. P. & Kopf M. Tissue-resident macrophages: guardians of organ homeostasis. Trends Immunol 42, 495–507 (2021). - PubMed
-
- Minutti C. M., Knipper J. A., Allen J. E. & Zaiss D. Tissue-specific contribution of macrophages to wound healing. Semin Cell Dev Biol 61, 3–11 (2017). - PubMed
-
- Vannella K. M. & Wynn T. A. Mechanisms of Organ Injury and Repair by Macrophages. Annual Review of Physiology 79, (2016). - PubMed
-
- Chakarov S. et al. Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 363, eaau0964 (2019). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
