This is a preprint.
C5aR1 antagonism suppresses inflammatory glial gene expression and alters cellular signaling in an aggressive Alzheimer's model
- PMID: 37662399
- PMCID: PMC10473603
- DOI: 10.1101/2023.08.22.554306
C5aR1 antagonism suppresses inflammatory glial gene expression and alters cellular signaling in an aggressive Alzheimer's model
Update in
-
C5aR1 antagonism suppresses inflammatory glial responses and alters cellular signaling in an Alzheimer's disease mouse model.Nat Commun. 2024 Aug 15;15(1):7028. doi: 10.1038/s41467-024-51163-6. Nat Commun. 2024. PMID: 39147742 Free PMC article.
Abstract
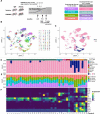
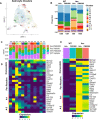
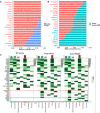
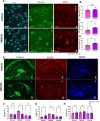
Alzheimer's disease (AD) is the leading cause of dementia in older adults, and the need for effective, sustainable therapeutic targets is imperative. Pharmacologic inhibition of C5aR1 reduces plaque load, gliosis and memory deficits in animal models. However, the cellular basis underlying this neuroprotection and which processes were the consequence of amyloid reduction vs alteration of the response to amyloid were unclear. In the Arctic model, the C5aR1 antagonist PMX205 did not reduce plaque load, but deficits in short-term memory in female mice were prevented. Hippocampal single cell and single nucleus RNA-seq clusters revealed C5aR1 dependent and independent gene expression and cell-cell communication. Microglial clusters containing neurotoxic disease-associated microglial genes were robustly upregulated in Arctic mice and drastically reduced with PMX205 treatment, while genes in microglia clusters that were overrepresented in the Arctic-PMX205 vs Arctic group were associated with synapse organization and transmission and learning. PMX205 treatment also reduced some A-1 astrocyte genes. In spite of changes in transcript levels, overall protein levels of some reactive glial markers were relatively unchanged by C5aR1 antagonism, as were clusters associated with protective responses to injury. C5aR1 inhibition promoted signaling pathways associated with cell growth and repair, such as TGFβ and FGF, in Arctic mice, while suppressing inflammatory pathways including PROS, Pecam1, and EPHA. In conclusion, pharmacologic C5aR1 inhibition prevents cognitive loss, limits microglial polarization to a detrimental inflammatory state and permits neuroprotective responses, as well as leaving protective functions of complement intact, making C5aR1 antagonism an attractive therapeutic strategy for individuals with AD.
Conflict of interest statement
Competing interests: Authors declare that they have no competing interests.
Figures



References
-
- 2022 Alzheimer’s disease facts and figures. Alzheimers Dement 18, 700–789 (2022). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
