Challenge of material haemocompatibility for microfluidic blood-contacting applications
- PMID: 37662438
- PMCID: PMC10469978
- DOI: 10.3389/fbioe.2023.1249753
Challenge of material haemocompatibility for microfluidic blood-contacting applications
Erratum in
-
Corrigendum: Challenge of material haemocompatibility for microfluidic blood-contacting applications.Front Bioeng Biotechnol. 2023 Oct 10;11:1297000. doi: 10.3389/fbioe.2023.1297000. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37885454 Free PMC article.
Abstract
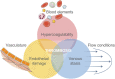
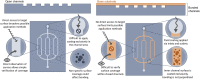
Biological applications of microfluidics technology is beginning to expand beyond the original focus of diagnostics, analytics and organ-on-chip devices. There is a growing interest in the development of microfluidic devices for therapeutic treatments, such as extra-corporeal haemodialysis and oxygenation. However, the great potential in this area comes with great challenges. Haemocompatibility of materials has long been a concern for blood-contacting medical devices, and microfluidic devices are no exception. The small channel size, high surface area to volume ratio and dynamic conditions integral to microchannels contribute to the blood-material interactions. This review will begin by describing features of microfluidic technology with a focus on blood-contacting applications. Material haemocompatibility will be discussed in the context of interactions with blood components, from the initial absorption of plasma proteins to the activation of cells and factors, and the contribution of these interactions to the coagulation cascade and thrombogenesis. Reference will be made to the testing requirements for medical devices in contact with blood, set out by International Standards in ISO 10993-4. Finally, we will review the techniques for improving microfluidic channel haemocompatibility through material surface modifications-including bioactive and biopassive coatings-and future directions.
Keywords: biomaterials; coating; haemocompatibility; medical devices; microfluidics; surface modification; thrombosis.
Copyright © 2023 Newman, Leclerc, Arditi, Calzuola, Feaugas, Roy, Porrini and Bechelany.
Conflict of interest statement
Authors GN, WA, SC, TF, ER, and CP were employed by the company Eden Tech. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ausri I. R., Feygin E. M., Cheng C. Q., Wang Y., Lin Z. Y. W., Tang X. S. (2018). A highly efficient and antifouling microfluidic platform for portable hemodialysis devices. MRS Commun. 8, 474–479. 10.1557/mrc.2018.43 - DOI
Publication types
LinkOut - more resources
Full Text Sources

