Evaluating the quality of studies reporting on clinical applications of stromal vascular fraction: A systematic review and proposed reporting guidelines (CLINIC-STRA-SVF)
- PMID: 37662694
- PMCID: PMC10474569
- DOI: 10.1016/j.reth.2023.08.003
Evaluating the quality of studies reporting on clinical applications of stromal vascular fraction: A systematic review and proposed reporting guidelines (CLINIC-STRA-SVF)
Abstract
Background: The stromal vascular fraction (SVF) has been widely explored in a number of therapeutic applications in several specialties. Its therapeutic potential is being increasingly demonstrated, although its mechanism of action is still unclear.
Objective: To evaluate the quality of studies reporting on clinical applications of SVF.
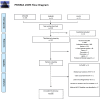
Method: This is a systematic literature review that followed the PRISMA guidelines with the search of the studies from December 1, 2012, to December 1, 2022, in the following databases: MEDLINE, LILACS and EMBASE. The level of evidence of the studies was assessed using the GRADE system, and the rigor used in the publication of the results was assessed in relation to adherence to the guidelines indicated by the EQUATOR Network Group. The CLINIC - STRA-SVF reporting guideline was developed after the completion of this systematic review.
Results: A total of 538 articles were found, and 77 articles were selected after reading the titles and abstracts and removing duplicates. Then, 15 studies were removed for not meeting the inclusion criteria, leaving 62 studies. The CLINIC - STRA-SVF was developed and consists of 33 items and two tables.
Conclusion: There is scientific evidence, although mostly with a low level of evidence, that the use of SVF in clinical applications is safe and effective. The information published in these studies should be standardized, and the CLINIC - STRA-SVF reporting guideline proposed in this study may assist in the design, conduct, recording and reporting of clinical trials and others clinical studies involving the SVF.
Keywords: Adipose-derived stem cells; Guideline; Regenerative medicine; Scientific rigor; Stromal vascular fraction.
© 2023 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
None.
Similar articles
-
Autologous adipose-derived stromal vascular fraction (SVF) in scar treatment among patients with keloids and hypertrophic scars: a systematic review and meta-analysis of current practices and outcomes.Am J Stem Cells. 2023 Dec 15;12(5):98-111. eCollection 2023. Am J Stem Cells. 2023. PMID: 38213639 Free PMC article. Review.
-
Systematic review: Advances of fat tissue engineering as bioactive scaffold, bioactive material, and source for adipose-derived mesenchymal stem cells in wound and scar treatment.Stem Cell Res Ther. 2021 Jun 2;12(1):318. doi: 10.1186/s13287-021-02397-4. Stem Cell Res Ther. 2021. PMID: 34078470 Free PMC article.
-
A systematic review of autologous adipose-derived stromal vascular fraction (SVF) for the treatment of acute cutaneous wounds.Arch Dermatol Res. 2022 Jul;314(5):417-425. doi: 10.1007/s00403-021-02242-x. Epub 2021 May 28. Arch Dermatol Res. 2022. PMID: 34047823
-
Adipose-derived stromal vascular fraction (SVF) in scar treatment: a systematic review protocol.Am J Stem Cells. 2022 Aug 20;11(4):56-63. eCollection 2022. Am J Stem Cells. 2022. PMID: 36189175 Free PMC article.
-
Clinical Efficacy of Bone Marrow Aspirate Concentrate Versus Stromal Vascular Fraction Injection in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis.Am J Sports Med. 2022 Apr;50(5):1451-1461. doi: 10.1177/03635465211014500. Epub 2021 Jun 8. Am J Sports Med. 2022. PMID: 34102078
Cited by
-
Efficacy of stromal vascular fraction for knee osteoarthritis: A prospective, single-centre, non-randomized study with 2 years follow-up.World J Orthop. 2024 May 18;15(5):457-468. doi: 10.5312/wjo.v15.i5.457. eCollection 2024 May 18. World J Orthop. 2024. PMID: 38835682 Free PMC article.
-
Treatment of medication-related osteonecrosis of the jaw with cell therapy.Front Cell Dev Biol. 2024 Jan 26;12:1338376. doi: 10.3389/fcell.2024.1338376. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38344747 Free PMC article.
-
Stromal vascular fraction in canine osteoarthritis: advantages, applications, and insights for veterinary practitioners.Front Vet Sci. 2025 May 16;12:1586629. doi: 10.3389/fvets.2025.1586629. eCollection 2025. Front Vet Sci. 2025. PMID: 40454169 Free PMC article. Review.
-
Challenges in the clinical translation of stromal vascular fraction therapy in regenerative medicine.World J Stem Cells. 2025 Jun 26;17(6):103775. doi: 10.4252/wjsc.v17.i6.103775. World J Stem Cells. 2025. PMID: 40585955 Free PMC article. Review.
-
Evidence-Based Clinical Practice Guidelines on Regenerative Medicine Treatment for Chronic Pain: A Consensus Report from a Multispecialty Working Group.J Pain Res. 2024 Sep 11;17:2951-3001. doi: 10.2147/JPR.S480559. eCollection 2024. J Pain Res. 2024. PMID: 39282657 Free PMC article. Review.
References
-
- Zuk P.A., Zhu M., Mizuno H., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. - PubMed
-
- XX Neuber G. Über die Wiederanheilung vollständig vom Körper getrennter, die ganze Fettschicht enthaltender Hautstücke. Zentralblatt Chir. 1893;30:16.
-
- Morestin H. Quelques cas de greffes graisseuse appliquees aIa chirurgie reparatrice. Bull Mem Soc Chir (Paris) 1915;41:1631.
-
- Rehman J., Traktuev D., Li J., et al. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109(10):1292–1298. - PubMed
Publication types
LinkOut - more resources
Full Text Sources