Producing polyglycerol polyester polyol for thermoplastic polyurethane application: A novel valorization of glycerol, a by-product of biodiesel production
- PMID: 37662775
- PMCID: PMC10472058
- DOI: 10.1016/j.heliyon.2023.e19491
Producing polyglycerol polyester polyol for thermoplastic polyurethane application: A novel valorization of glycerol, a by-product of biodiesel production
Abstract
The production of biodiesel generates glycerol as a by-product that needs valorization. Glycerol, when converted to polyglycerol, is a potential polyol for bio-based thermoplastic polyurethane (TPU) production. In this study, a novel polyglycerol polyester polyol (PPP) was developed from refined glycerol and coconut oil-based polyester polyol. Glycerol was first converted to glycerol acetate and then polymerized with coconut oil-based polyester polyol (CPP) as secondary polyol and phthalic anhydride. The resulting PPP polymerized at 220 °C and OH:COOH molar ratio of 2.5 exhibited an OH number of <100 mg KOH·g sample-1, an acid number of <10 mg KOH·g sample-1, and a molecular weight (MW) of 3697 g mol-1 meeting the polyol requirement properties for TPU (Handlin et al., 2001; Parcheta et al., 2020) [1-2]. Fourier-transform infrared (FTIR) spectroscopic characterization determined that higher reaction temperatures increase the polymerization rate and decrease the OH and acid numbers. Further, higher OH:COOH molar ratios decrease the polymerization rate and acid number, and increase the OH number. Gel permeation chromatography determined the molecular weight of PPP and suggested two distinct molecular structures which differ only in the number of moles of CPP in the structure. A differential scanning calorimetric (DSC) experiment on a sample of PPP-based polyurethane revealed that it was able to melt and remelt after 3 heating cycles which demonstrates its thermoplastic ability. The novel PPP derived from the glycerol by-product of biodiesel industries can potentially replace petroleum-derived polyols for TPU production.
Keywords: Bio-based; Coconut oil-based polyol; Glycerol; Polyglycerol; Thermoplastic polyurethane.
© 2023 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
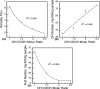
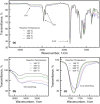
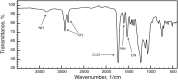
 O absorption (c) of the ester groups formed by the reaction of alcohol and carboxylic acid groups of the polyglycerol polyester polyol synthesized using different reaction temperatures (160, 180, 200 and 220 °C) and an OH:COOH molar ratio of 1.0 for 4 h.
O absorption (c) of the ester groups formed by the reaction of alcohol and carboxylic acid groups of the polyglycerol polyester polyol synthesized using different reaction temperatures (160, 180, 200 and 220 °C) and an OH:COOH molar ratio of 1.0 for 4 h.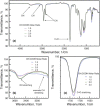
 O absorption (c) of the ester groups formed by the reaction of alcohol and carboxylic acid groups of the polyglycerol polyester polyol synthesized using different OH:COOH molar ratios (1.0, 1.5, 2.0, and 2.5) and a reaction temperature of 220 °C for 4 h.
O absorption (c) of the ester groups formed by the reaction of alcohol and carboxylic acid groups of the polyglycerol polyester polyol synthesized using different OH:COOH molar ratios (1.0, 1.5, 2.0, and 2.5) and a reaction temperature of 220 °C for 4 h.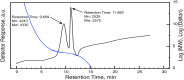



Similar articles
-
Flexible Polyurethane Foams Modified with Novel Coconut Monoglycerides-Based Polyester Polyols.ACS Omega. 2024 Jan 15;9(4):4497-4512. doi: 10.1021/acsomega.3c07312. eCollection 2024 Jan 30. ACS Omega. 2024. PMID: 38313545 Free PMC article.
-
Utilization of microbial oil obtained from crude glycerol for the production of polyol and its subsequent conversion to polyurethane foams.Bioresour Technol. 2017 Jul;235:309-315. doi: 10.1016/j.biortech.2017.03.126. Epub 2017 Mar 24. Bioresour Technol. 2017. PMID: 28371769
-
Production of Bio-Based Polyol from Coconut Fatty Acid Distillate (CFAD) and Crude Glycerol for Rigid Polyurethane Foam Applications.Materials (Basel). 2023 Aug 3;16(15):5453. doi: 10.3390/ma16155453. Materials (Basel). 2023. PMID: 37570156 Free PMC article.
-
Synthesis of Thermoplastic Polyurethanes Containing Bio-Based Polyester Polyol and Their Fiber Property.Polymers (Basel). 2022 May 16;14(10):2033. doi: 10.3390/polym14102033. Polymers (Basel). 2022. PMID: 35631915 Free PMC article.
-
Biochemistry, genetics and biotechnology of glycerol utilization in Pseudomonas species.Microb Biotechnol. 2020 Jan;13(1):32-53. doi: 10.1111/1751-7915.13400. Epub 2019 Mar 18. Microb Biotechnol. 2020. PMID: 30883020 Free PMC article. Review.
Cited by
-
Vegetable Oils for Material Applications - Available Biobased Compounds Seeking Their Utilities.ACS Polym Au. 2025 Mar 19;5(2):105-128. doi: 10.1021/acspolymersau.5c00001. eCollection 2025 Apr 9. ACS Polym Au. 2025. PMID: 40226347 Free PMC article. Review.
-
Techno-Environmental Assessment of Biodiesel Production from Acid Oil via One-Step and Two-Step Esterification.ACS Omega. 2025 May 22;10(21):21280-21291. doi: 10.1021/acsomega.4c10777. eCollection 2025 Jun 3. ACS Omega. 2025. PMID: 40487993 Free PMC article.
References
-
- Parcheta P., Głowińska E., Datta J. Effect of bio-based components on the chemical structure, thermal stability and mechanical properties of green thermoplastic polyurethane elastomers. Eur. Polym. J. 2020;123 doi: 10.1016/j.eurpolymj.2019.109422. - DOI
-
- Chozhavendhan S., Praveen Kumar R., Elavazhagan S., Barathiraja B., Jayakumar M., Varjani S.J. In: Waste to Wealth, Energy, Environment, and Sustainability. Singhania R.R., Agarwal R.A., Kumar R.P., Sukumaran R.K., editors. Springer Singapore; Singapore: 2018. Utilization of crude glycerol from biodiesel industry for the production of value-added bioproducts; pp. 65–82. - DOI
-
- Major Biodiesel Producing Countries 2021. 2021. https://www.statista.com/statistics/271472/biodiesel-production-in-selec... [WWW Document] Statista. URL. 1.25.23.
LinkOut - more resources
Full Text Sources

