Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-COV-2: similarities and differences
- PMID: 37662901
- PMCID: PMC10470049
- DOI: 10.3389/fimmu.2023.1244556
Initial immune response after exposure to Mycobacterium tuberculosis or to SARS-COV-2: similarities and differences
Abstract
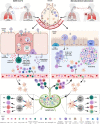
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb) and Coronavirus disease-2019 (COVID-19), whose etiologic agent is severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), are currently the two deadliest infectious diseases in humans, which together have caused about more than 11 million deaths worldwide in the past 3 years. TB and COVID-19 share several aspects including the droplet- and aerosol-borne transmissibility, the lungs as primary target, some symptoms, and diagnostic tools. However, these two infectious diseases differ in other aspects as their incubation period, immune cells involved, persistence and the immunopathological response. In this review, we highlight the similarities and differences between TB and COVID-19 focusing on the innate and adaptive immune response induced after the exposure to Mtb and SARS-CoV-2 and the pathological pathways linking the two infections. Moreover, we provide a brief overview of the immune response in case of TB-COVID-19 co-infection highlighting the similarities and differences of each individual infection. A comprehensive understanding of the immune response involved in TB and COVID-19 is of utmost importance for the design of effective therapeutic strategies and vaccines for both diseases.
Keywords: COVID-19; M. tuberculosis; SARS-CoV-2; T cell response; antibody response; co-infection; innate response; tuberculosis.
Copyright © 2023 Aiello, Najafi-Fard and Goletti.
Conflict of interest statement
Author DG has been a member of the advisory board of Biomerieux and Eli Lilly in 2020 and 2021 and is currently scientific advisor of PDB Biotec. She received fees for educational training or consultancy from Almirall, Biogen, Celgene, Diasorin, Janssen, Qiagen and Quidel. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- WHO . Coronavirus disease (COVID-19) – World Health Organization (2023). Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed June 13, 2023).
-
- Global tuberculosis report 2022 . Available at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/globa... (Accessed June 19, 2023).
-
- Aiello A, Grossi A, Meschi S, Meledandri M, Vanini V, Petrone L, et al. . Coordinated innate and T-cell immune responses in mild COVID-19 patients from household contacts of COVID-19 cases during the first pandemic wave. Front Immunol (2022) 13:920227. doi: 10.3389/fimmu.2022.920227 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

