Immune cells as messengers from the CNS to the periphery: the role of the meningeal lymphatic system in immune cell migration from the CNS
- PMID: 37662908
- PMCID: PMC10471710
- DOI: 10.3389/fimmu.2023.1233908
Immune cells as messengers from the CNS to the periphery: the role of the meningeal lymphatic system in immune cell migration from the CNS
Abstract
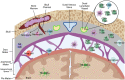
In recent decades there has been a large focus on understanding the mechanisms of peripheral immune cell infiltration into the central nervous system (CNS) in neuroinflammatory diseases. This intense research led to several immunomodulatory therapies to attempt to regulate immune cell infiltration at the blood brain barrier (BBB), the choroid plexus (ChP) epithelium, and the glial barrier. The fate of these infiltrating immune cells depends on both the neuroinflammatory environment and their type-specific interactions with innate cells of the CNS. Although the fate of the majority of tissue infiltrating immune cells is death, a percentage of these cells could become tissue resident immune cells. Additionally, key populations of immune cells can possess the ability to "drain" out of the CNS and act as messengers reporting signals from the CNS toward peripheral lymphatics. Recent data supports that the meningeal lymphatic system is involved not just in fluid homeostatic functions in the CNS but also in facilitating immune cell migration, most notably dendritic cell migration from the CNS to the meningeal borders and to the draining cervical lymph nodes. Similar to the peripheral sites, draining immune cells from the CNS during neuroinflammation have the potential to coordinate immunity in the lymph nodes and thus influence disease. Here in this review, we will evaluate evidence of immune cell drainage from the brain via the meningeal lymphatics and establish the importance of this in animal models and humans. We will discuss how targeting immune cells at sites like the meningeal lymphatics could provide a new mechanism to better provide treatment for a variety of neurological conditions.
Keywords: CNS; cervical lymph node; cribriform plate; dendritic cells; immune cell migration; meningeal lymphatics; olfactory nerves.
Copyright © 2023 Laaker, Baenen, Kovács, Sandor and Fabry.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Beuker C, Strecker JK, Rawal R, Schmidt-Pogoda A, Ruck T, Wiendl H, et al. Immune cell infiltration into the brain after ischemic stroke in humans compared to mice and rats: a systematic review and meta-analysis. Transl Stroke Res (2021) 12(6):976–90. doi: 10.1007/s12975-021-00887-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

