Characterization of a fatal feline panleukopenia virus derived from giant panda with broad cell tropism and zoonotic potential
- PMID: 37662912
- PMCID: PMC10469695
- DOI: 10.3389/fimmu.2023.1237630
Characterization of a fatal feline panleukopenia virus derived from giant panda with broad cell tropism and zoonotic potential
Abstract
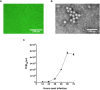
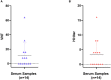
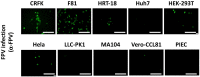
Represented by feline panleukopenia virus (FPV) and canine parvovirus (CPV), the species carnivore protoparvovirus 1 has a worldwide distribution through continuous ci13rculation in companion animals such as cats and dogs. Subsequently, both FPV and CPV had engaged in host-to-host transfer to other wild animal hosts of the order Carnivora. In the present study, we emphasized the significance of cross-species transmission of parvoviruses with the isolation and characterization of an FPV from giant panda displaying severe and fatal symptoms. The isolated virus, designated pFPV-sc, displayed similar morphology as FPV, while phylogenetic analysis indicated that the nucleotide sequence of pFPV-sc clades with Chinese FPV isolates. Despite pFPV-sc is seemingly an outcome of a spillover infection event from domestic cats to giant pandas, our study also provided serological evidence that FPV or other parvoviruses closely related to FPV could be already prevalent in giant pandas in 2011. Initiation of host transfer of pFPV-sc is likely with association to giant panda transferrin receptor (TfR), as TfR of giant panda shares high homology with feline TfR. Strikingly, our data also indicate that pFPV-sc can infect cell lines of other mammal species, including humans. To sum up, observations from this study shall promote future research of cross-host transmission and antiviral intervention of Carnivore protoparvovirus 1, and necessitate surveillance studies in thus far unacknowledged potential reservoirs.
Keywords: TfR; cross-species transmission; fatal; feline panleukopenia virus; giant panda.
Copyright © 2023 Zhao, Hu, Lan, Yang, Peng, Yan, Luo, Wu, Lang and Yan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

