DNA and histones impair the mechanical stability and lytic susceptibility of fibrin formed by staphylocoagulase
- PMID: 37662916
- PMCID: PMC10470048
- DOI: 10.3389/fimmu.2023.1233128
DNA and histones impair the mechanical stability and lytic susceptibility of fibrin formed by staphylocoagulase
Abstract
Background: Staphylocoagulase (SCG) is a virulence factor of Staphylococcus aureus, one of the most lethal pathogens of our times. The complex of SCG with prothrombin (SCG/ProT) can clot fibrinogen, and SCG/ProT-induced fibrin and plasma clots have been described to show decreased mechanical and lytic resistance, which may contribute to septic emboli from infected cardiac vegetations. At infection sites, neutrophils can release DNA and histones, as parts of neutrophil extracellular traps (NETs), which in turn favor thrombosis, inhibit fibrinolysis and strengthen clot structure.
Objectives: To characterize the combined effects of major NET-components (DNA, histone H1 and H3) on SCG/ProT-induced clot structure, mechanical and lytic stability.
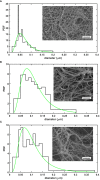
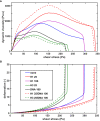
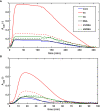
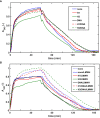
Methods: Recombinant SCG was used to clot purified fibrinogen and plasma. The kinetics of formation and lysis of fibrin and plasma clots containing H1 or core histones+/-DNA were followed by turbidimetry. Fibrin structure and mechanical stability were characterized with scanning electron microscopy, pressure-driven permeation, and oscillation rheometry.
Results: Histones and DNA favored the formation of thicker fibrin fibers and a more heterogeneous clot structure including high porosity with H1 histone, whereas low porosity with core histones and DNA. As opposed to previous observations with thrombin-induced clots, SCG/ProT-induced fibrin was not mechanically stabilized by histones. Similarly to thrombin-induced clots, the DNA-histone complexes prolonged fibrinolysis with tissue-type plasminogen activator (up to 2-fold). The anti-fibrinolytic effect of the DNA and DNA-H3 complex was observed in plasma clots too. Heparin (low molecular weight) accelerated the lysis of SCG/ProT-clots from plasma, even if DNA and histones were also present.
Conclusions: In the interplay of NETs and fibrin formed by SCG, DNA and histones promote structural heterogeneity in the clots, and fail to stabilize them against mechanical stress. The DNA-histone complexes render the SCG-fibrin more resistant to lysis and thereby less prone to embolization.
Keywords: NET; extracellular DNA; fibrin; fibrinolysis; histone; staphylocoagulase.
Copyright © 2023 Komorowicz, Farkas, Szabó, Cherrington, Thelwell and Kolev.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ostregaard L, Voldstedlund M, Bruun NE, Bundgaard H, Iversen K, Kober N, et al. Temporal changes, patient characteristics, and mortality, according to microbiological cause of infective endocarditis: a nationwide study. J Am Heart Assoc (2022) 11:025801. doi: 10.1161/JAHA.122.025801 - DOI - PMC - PubMed
-
- Garcia-Cabrera E, Fernandez-Hidalgo N, AlmIrante B, Ivanova-Georgieva R, Noureddine M, Plata A, et al. Neurological complications of infective endocarditis. Risk factors, Outcome, and Impact of cardiac surgery: a multicenter observational study. Circulation (2013) 127:2272–84. doi: 10.1161/CIRCULATIONAHA.112.000813 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

