Immune responses to Neisseria gonorrhoeae and implications for vaccine development
- PMID: 37662926
- PMCID: PMC10470030
- DOI: 10.3389/fimmu.2023.1248613
Immune responses to Neisseria gonorrhoeae and implications for vaccine development
Abstract
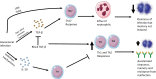
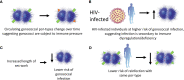
Neisseria gonorrheoae is the causative agent of gonorrhea, a sexually transmitted infection responsible for a major burden of disease with a high global prevalence. Protective immunity to infection is often not observed in humans, possible due to high variability of key antigens, induction of blocking antibodies, or a large number of infections being relatively superficial and not inducing a strong immune response. N. gonorrhoeae is a strictly human pathogen, however, studies using mouse models provide useful insights into the immune response to gonorrhea. In mice, N. gonorrhoea appears to avoid a protective Th1 response by inducing a less protective Th17 response. In mouse models, candidate vaccines which provoke a Th1 response can accelerate the clearance of gonococcus from the mouse female genital tract. Human studies indicate that natural infection often induces a limited immune response, with modest antibody responses, which may correlate with the clinical severity of gonococcal disease. Studies of cytokine responses to gonococcal infection in humans provide conflicting evidence as to whether infection induces an IL-17 response. However, there is evidence for limited induction of protective immunity from a study of female sex workers in Kenya. A controlled human infection model (CHIM) has been used to examine the immune response to gonococcal infection in male volunteers, but has not to date demonstrated protection against re-infection. Correlates of protection for gonorrhea are lacking, which has hampered the progress towards developing a successful vaccine. However, the finding that the Neisseria meningitidis serogroup B vaccines, elicit cross-protection against gonorrhea has invigorated the gonococcal vaccine field. More studies of infection in humans, either natural infection or CHIM studies, are needed to understand better gonococcal protective immunity.
Keywords: Neisseria; STI; gonococcus; gonorrhea; immunology; vaccines.
Copyright © 2023 Belcher, Rollier, Dold, Ross and MacLennan.
Conflict of interest statement
Author JR reports personal fees from GSK Pharma; ownership of shares in GSK Pharma and AstraZeneca Pharma; lead author of the UK and European Guidelines on Pelvic Inflammatory Disease; Member of the European Sexually Transmitted Infections Guidelines Editorial Board. He is a UK National Institute for Health Research Journals Editor and associate editor of Sexually Transmitted Infections journal. He is treasurer for the International Union against Sexually Transmitted Infections and chair of charity trustees for the Sexually Transmitted Infections Research Foundation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Joseph Davey DL, Shull HI, Billings JD, Wang D, Adachi K, Klausner JD. Prevalence of curable sexually transmitted infections in pregnant women in low- and middle-income countries from 2010 to 2015: A systematic review. Sex Transm Dis (2016) 43(7):450–8. doi: 10.1097/OLQ.0000000000000460 - DOI - PMC - PubMed
-
- Zhang L, Zhang C, Zeng Y, Li Y, Huang S, Wang F, et al. Emergence and characterization of a ceftriaxone-resistant neisseria gonorrhoeae FC428 clone evolving moderate-level resistance to azithromycin in Shenzhen, China. Infect Drug Resist (2021) 14:4271–6. doi: 10.2147/IDR.S336212 - DOI - PMC - PubMed
-
- Kueakulpattana N, Wannigama DL, Luk-In S, Hongsing P, Hurst C, Badavath VN, et al. Multidrug-resistant Neisseria gonorrhoeae infection in heterosexual men with reduced susceptibility to ceftriaxone, first report in Thailand. Sci Rep (2021) 11(1):21659. doi: 10.1038/s41598-021-00675-y - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

