Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: a cohort study from the Fukushima vaccination community survey, Japan
- PMID: 37662950
- PMCID: PMC10469480
- DOI: 10.3389/fimmu.2023.1240425
Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: a cohort study from the Fukushima vaccination community survey, Japan
Abstract
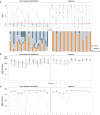
The bivalent mRNA vaccine is recommended to address coronavirus disease variants, with additional doses suggested for high-risk groups. However, the effectiveness, optimal frequency, and number of doses remain uncertain. In this study, we examined the long-term cellular and humoral immune responses following the fifth administration of the mRNA severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine in patients undergoing hemodialysis. To our knowledge, this is the first study to monitor long-term data on humoral and cellular immunity dynamics in high-risk populations after five doses of mRNA vaccination, including the bivalent mRNA vaccine. Whereas most patients maintained humoral immunity throughout the observation period, we observed reduced cellular immune reactivity as measured by the ancestral-strain-stimulated ELISpot assay in a subset of patients. Half of the individuals (50%; 14/28) maintained cellular immunity three months after the fifth dose, despite acquiring humoral immunity. The absence of a relationship between positive controls and T-Spot reactivity suggests that these immune alterations were specific to SARS-CoV-2. In multivariable analysis, participants aged ≥70 years showed a marginally significant lower likelihood of having reactive results. Notably, among the 14 individuals who received heterologous vaccines, 13 successfully acquired cellular immunity, supporting the effectiveness of this administration strategy. These findings provide valuable insights for future vaccination strategies in vulnerable populations. However, further research is needed to evaluate the involvement of immune tolerance and exhaustion through repeated vaccination to optimize immunization strategies.
Keywords: SARS-CoV2; cellular immunity; dialysis patient; immune imprinting; vaccination; vulnerable population.
Copyright © 2023 Tani, Takita, Wakui, Saito, Nishiuchi, Zhao, Yamamoto, Kawamura, Sugiyama, Nakayama, Kaneko, Kodama, Shinaha and Tsubokura.
Conflict of interest statement
YK is employed by company Medical & Biological Laboratories, Co., Ltd., Tokyo, Japan. MBL imported the testing material used in this research. YK and MTs received a grant from Pfizer Health Research Foundation for research unrelated to this work. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Centers for Disease Control and Prevention (CDC) . Coronavirus (COVID-19) Update: FDA Authorizes Moderna, Pfizer-BioNTech Bivalent COVID-19 Vaccines for Use as a Booster Dose (2022). Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19....
-
- Centers for Disease Control and Prevention (CDC) . Interim Clinical Considerations for Use of COVID-19 Vaccines in the United States (2023). Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-co....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

