Temporal patterns of cytokine and injury biomarkers in hospitalized COVID-19 patients treated with methylprednisolone
- PMID: 37662953
- PMCID: PMC10468998
- DOI: 10.3389/fimmu.2023.1229611
Temporal patterns of cytokine and injury biomarkers in hospitalized COVID-19 patients treated with methylprednisolone
Abstract
Background: The novel coronavirus disease 2019 (COVID-19) presents with complex pathophysiological effects in various organ systems. Following the COVID-19, there are shifts in biomarker and cytokine equilibrium associated with altered physiological processes arising from viral damage or aggressive immunological response. We hypothesized that high daily dose methylprednisolone improved the injury biomarkers and serum cytokine profiles in COVID-19 patients.
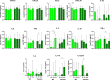
Methods: Injury biomarker and cytokine analysis was performed on 50 SARS-Cov-2 negative controls and 101 hospitalized severe COVID-19 patients: 49 methylprednisolone-treated (MP group) and 52 placebo-treated serum samples. Samples from the treated groups collected on days D1 (pre-treatment) all the groups, D7 (2 days after ending therapy) and D14 were analyzed. Luminex assay quantified the biomarkers HMGB1, FABP3, myoglobin, troponin I and NTproBNP. Immune mediators (CXCL8, CCL2, CXCL9, CXCL10, TNF, IFN-γ, IL-17A, IL-12p70, IL-10, IL-6, IL-4, IL-2, and IL-1β) were quantified using cytometric bead array.
Results: At pretreatment, the two treatment groups were comparable demographically. At pre-treatment (D1), injury biomarkers (HMGB1, TnI, myoglobin and FABP3) were distinctly elevated. At D7, HMGB1 was significantly higher in the MP group (p=0.0448) compared to the placebo group, while HMGB1 in the placebo group diminished significantly by D14 (p=0.0115). Compared to healthy control samples, several immune mediators (IL-17A, IL-6, IL-10, MIG, MCP-1, and IP-10) were considerably elevated at baseline (all p≤0.05). At D7, MIG and IP-10 of the MP-group were significantly lower than in the placebo-group (p=0.0431, p=0.0069, respectively). Longitudinally, IL-2 (MP-group) and IL-17A (placebo-group) had increased significantly by D14. In placebo group, IL-2 and IL-17A continuously increased, as IL-12p70, IL-10 and IP-10 steadily decreased during follow-up. The MP treated group had IL-2, IFN-γ, IL-17A and IL-12p70 progressively increase while IL-1β and IL-10 gradually decreased towards D14. Moderate to strong positive correlations between chemokines and cytokines were observed on D7 and D14.
Conclusion: These findings suggest MP treatment could ameliorate levels of myoglobin and FABP3, but appeared to have no impact on HMGB1, TnI and NTproBNP. In addition, methylprednisolone relieves the COVID-19 induced inflammatory response by diminishing MIG and IP-10 levels. Overall, corticosteroid (methylprednisolone) use in COVID-19 management influences the immunological molecule and injury biomarker profile in COVID-19 patients.
Keywords: SARS-CoV-2; biomarkers; cytokine; immune mediators; injury; methylprednisolone.
Copyright © 2023 Mwangi, Netto, de Morais, Silva, Silva, Lima, Neves, Borba, Val, de Almeida, Costa, Sampaio, Gardinassi, de Lacerda, Monteiro and de Melo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


 ), Placebo (
), Placebo ( ) and MP group (
) and MP group ( ). The different line sizes and types demonstrate the interrelationships between the chemokines and cytokines circulating in the peripheral blood from the different study groups. Dashed lines between molecules indicate a negative correlation, while solid lines indicate a positive correlation. The thickness of these indicates the strength of the correlation. The correlation index (r) used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r≥0.36 to r ≤ 0.67) or strong (r≥0.68).
). The different line sizes and types demonstrate the interrelationships between the chemokines and cytokines circulating in the peripheral blood from the different study groups. Dashed lines between molecules indicate a negative correlation, while solid lines indicate a positive correlation. The thickness of these indicates the strength of the correlation. The correlation index (r) used to categorize the strength of the correlation as weak (r ≤ 0.35), moderate (r≥0.36 to r ≤ 0.67) or strong (r≥0.68).References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

