Therapeutic cancer vaccination against mutant calreticulin in myeloproliferative neoplasms induces expansion of specific T cells in the periphery but specific T cells fail to enrich in the bone marrow
- PMID: 37662956
- PMCID: PMC10470021
- DOI: 10.3389/fimmu.2023.1240678
Therapeutic cancer vaccination against mutant calreticulin in myeloproliferative neoplasms induces expansion of specific T cells in the periphery but specific T cells fail to enrich in the bone marrow
Abstract
Background: Therapeutic cancer vaccination against mutant calreticulin (CALR) in patients with CALR-mutant (CALRmut) myeloproliferative neoplasms (MPN) induces strong T-cell responses against mutant CALR yet fails to demonstrate clinical activity. Infiltration of tumor specific T cells into the tumor microenvironment is needed to attain a clinical response to therapeutic cancer vaccination.
Aim: Determine if CALRmut specific T cells isolated from vaccinated patients enrich in the bone marrow upon completion of vaccination and explore possible explanations for the lack of enrichment.
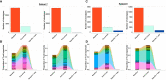
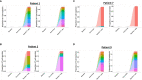
Methods: CALRmut specific T cells from four of ten vaccinated patients were expanded, enriched, and analyzed by T-cell receptor sequencing (TCRSeq). The TCRs identified were used as fingerprints of CALRmut specific T cells. Bone marrow aspirations from the four patients were acquired at baseline and at the end of trial. T cells were enriched from the bone marrow aspirations and analyzed by TCRSeq to identify the presence and fraction of CALRmut specific T cells at the two different time points. In silico calculations were performed to calculate the ratio between transformed cells and effector cells in patients with CALRmut MPN.
Results: The fraction of CALRmut specific T cells in the bone marrow did not increase upon completion of the vaccination trial. In general, the T cell repertoire in the bone marrow remains relatively constant through the vaccination trial. The enriched and expanded CALRmut specific T cells recognize peripheral blood autologous CALRmut cells. In silico analyses demonstrate a high imbalance in the fraction of CALRmut cells and CALRmut specific effector T-cells in peripheral blood.
Conclusion: CALRmut specific T cells do not enrich in the bone marrow after therapeutic cancer peptide vaccination against mutant CALR. The specific T cells recognize autologous peripheral blood derived CALRmut cells. In silico analyses demonstrate a high imbalance between the number of transformed cells and CALRmut specific effector T-cells in the periphery. We suggest that the high burden of transformed cells in the periphery compared to the number of effector cells could impact the ability of specific T cells to enrich in the bone marrow.
Keywords: adaptive immunity; calreticulin; cancer vaccines; immune escape; myeloproliferative neoplasms.
Copyright © 2023 Holmström, Andersen, Traynor, Ahmad, Lisle, Handlos Grauslund, Skov, Kjær, Ottesen, Gjerstorff, Hasselbalch and Andersen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

