Leverage of nuclease-deficient CasX for preventing pathological angiogenesis
- PMID: 37662968
- PMCID: PMC10469388
- DOI: 10.1016/j.omtn.2023.08.001
Leverage of nuclease-deficient CasX for preventing pathological angiogenesis
Abstract
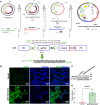
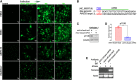
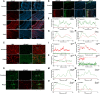
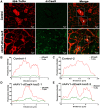
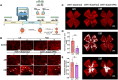
Gene editing with a CRISPR/Cas system is a novel potential strategy for treating human diseases. Pharmacological inhibition of phosphoinositide 3-kinase (PI3K) δ suppresses retinal angiogenesis in a mouse model of oxygen-induced retinopathy. Here we show that an innovative system of adeno-associated virus (AAV)-mediated CRISPR/nuclease-deficient (d)CasX fused with the Krueppel-associated box (KRAB) domain is leveraged to block (81.2% ± 6.5%) in vitro expression of p110δ, the catalytic subunit of PI3Kδ, encoded by Pik3cd. This CRISPR/dCasX-KRAB (4, 269 bp) system is small enough to be fit into a single AAV vector. We then document that recombinant AAV serotype (rAAV)1 efficiently transduces vascular endothelial cells from pathologic retinal vessels, which show high expression of p110δ; furthermore, we demonstrate that blockade of retinal p110δ expression by intravitreally injected rAAV1-CRISPR/dCasX-KRAB targeting the Pik3cd promoter prevents (32.1% ± 5.3%) retinal p110δ expression as well as pathological retinal angiogenesis in a mouse model of oxygen-induced retinopathy. These data establish a strong foundation for treating pathological angiogenesis by AAV-mediated CRISPR interference with p110δ expression.
Keywords: CRISPR/dCasX-KRAB; MT: RNA/DNA editing; PI3Kδ; Pik3cd; angiogenesis; oxygen-induced retinopathy; rAAV1.
© 2023 The Author(s).
Conflict of interest statement
All the authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Šuleková M., Fitzgerald K.T. Can the Thought of Teilhard de Chardin Carry Us Past Current Contentious Discussions of Gene Editing Technologies? Camb. Q. Healthc. Ethics. 2019;28:62–75. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

