Cryptosporidium parvum-induced neutrophil extracellular traps in neonatal calves is a stage-independent process
- PMID: 37662980
- PMCID: PMC10470472
- DOI: 10.3389/fvets.2023.1256726
Cryptosporidium parvum-induced neutrophil extracellular traps in neonatal calves is a stage-independent process
Abstract
Introduction: Infections with the apicomplexan obligate intracellular parasite Cryptosporidium parvum lead to cryptosporidiosis-a worldwide zoonotic infection. C. parvum is one of the most common diarrheal pathogens in young calves, which are the main reservoir of the pathogen. Cryptosporidiosis leads to severe economic losses in the calf industry and being a major contributor to diarrhea morbidity and mortality in children. Polymorphonuclear neutrophils (PMN) are part of the innate immune system. Their effector mechanisms directed against invasive parasites include phagocytosis, production of antimicrobial molecules as well as the formation of so-called neutrophil extracellular traps (NETs). Like other leukocytes of the innate immune system, PMN are thus able to release chromatin fibers enriched with antimicrobial granular molecules extracellularly thereby immobilizing and partially killing invasive bacteria, viruses, fungi and parasites.
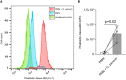
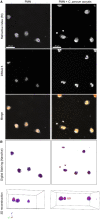
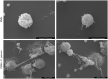
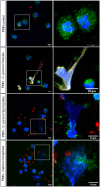
Methods: In vitro interactions of neonatal bovine PMN and C. parvum-oocysts and sporozoites were illustrated microscopically via scanning electron microscopy- and live cell imaging 3D holotomographic microscopy analyses. C. parvum-triggered NETosis was quantified via extracellular DNA measurements as well as verified via detection of NET-typical molecules [histones, neutrophil elastase (NE)] through immunofluorescence microscopy analysis. To verify the role of ATP in neonatal-derived NETosis, inhibition experiments were performed with NF449 (purinergic receptor antagonist with high specificity to P2X1 receptor).
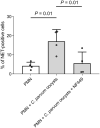
Results and discussion: Using immunofluorescence- and SEM-based analyses, we demonstrate here for the first time that neonate bovine PMN are capable of forming NETs against C. parvum-sporozoites and oocysts, thus as a stage-independent cell death process. Our data further showed that C. parvum strongly induces suicidal neonatal NETosis in a P2X1-dependent manner, suggesting anti-cryptosporidial effects not only through firm sporozoite ensnarement and hampered sporozoite excystation, but also via direct exposure to NETs-associated toxic components.
Keywords: Cryptosporidium parvum; NETosis; calves; neonates; polymorphonuclear neutrophils.
Copyright © 2023 Grabbe, Conejeros, Velásquez, Hasheminasab, Kamena, Wehrend, Gärtner, Taubert and Hermosilla.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. (2013) 382:209–22. 10.1016/S0140-6736(13)60844-2 - DOI - PubMed
LinkOut - more resources
Full Text Sources

