Vasopressin versus epinephrine during cardiopulmonary resuscitation of asphyxiated newborns: A study protocol for a prospective, cluster, open label, single-center, randomized controlled phase 2 trial - The VERSE-Trial
- PMID: 37663146
- PMCID: PMC10474318
- DOI: 10.1016/j.resplu.2023.100459
Vasopressin versus epinephrine during cardiopulmonary resuscitation of asphyxiated newborns: A study protocol for a prospective, cluster, open label, single-center, randomized controlled phase 2 trial - The VERSE-Trial
Abstract
Introduction: Current neonatal resuscitation guidelines recommend the use of epinephrine during neonatal cardiopulmonary resuscitation (CPR). However, newborns receiving epinephrine continue to have high rates of mortality and neurodevelopmental disability. The infrequent need for neonatal CPR, coupled with an inability to consistently anticipate which newborn infants are at risk of requiring CPR, explains the lack of high-quality evidence (i.e., large randomized clinical trials) to better guide healthcare providers in their resuscitative effort. Therefore, we need neonatal data to determine the optimal vasopressor therapy during neonatal CPR. The current pilot trial will examine the efficacy of vasopressin versus epinephrine during CPR of asphyxiated newborn infants.
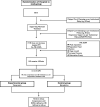
Methods and analysis: The trial will be a prospective, cluster, open label, single-center, randomized controlled trial on two alternative cardiovascular supportive medications. This study will assess the primary outcome of time to return of spontaneous circulation (ROSC) in newborns requiring CPR in the delivery room who were treated with either vasopressin (intervention) or epinephrine (control). Secondary outcomes such as infant mortality and other clinical outcome measures will also be collected. An estimated 20 newborns will be recruited, and comparisons will be made between asphyxiated infants treated with either drugs.
Ethics and dissemination: This study has been approved by the Research Ethics Board at the University of Alberta (June 16, 2023). Study findings will be published in peer-reviewed journals, presented at conferences, and communicated to relevant participants and stakeholders.Trial registration: ClinicalTrial.gov Identifier: NCT05738148. Registered February 21, 2023.
Keywords: Adrenaline; Asphyxia; CHEST compressions; Epinephrine; Infants; Neonatal resuscitation; Newborn; Vasopressin.
© 2023 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Similar articles
-
The SURV1VE trial-sustained inflation and chest compression versus 3:1 chest compression-to-ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: study protocol for a cluster randomized controlled trial.Trials. 2019 Feb 19;20(1):139. doi: 10.1186/s13063-019-3240-8. Trials. 2019. PMID: 30782199 Free PMC article.
-
Vasopressin versus epinephrine during neonatal cardiopulmonary resuscitation of asphyxiated post-transitional piglets.Resusc Plus. 2023 Jul 17;15:100427. doi: 10.1016/j.resplu.2023.100427. eCollection 2023 Sep. Resusc Plus. 2023. PMID: 37519409 Free PMC article.
-
2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric basic life support.Pediatrics. 2006 May;117(5):e989-1004. doi: 10.1542/peds.2006-0219. Pediatrics. 2006. PMID: 16651298
-
Cardiac Agents during Neonatal Cardiopulmonary Resuscitation.Neonatology. 2024;121(2):157-166. doi: 10.1159/000535502. Epub 2024 Jan 16. Neonatology. 2024. PMID: 38228124 Review.
-
Evidence for vasopressors during cardiopulmonary resuscitation in newborn infants.Minerva Pediatr. 2019 Apr;71(2):159-173. doi: 10.23736/S0026-4946.18.05452-X. Epub 2018 Dec 3. Minerva Pediatr. 2019. PMID: 30511562 Review.
Cited by
-
The current clinical landscape of preterm infants less than 32 weeks of gestation receiving delivery room chest compression in Jiangsu Province, China.Resusc Plus. 2025 Feb 13;22:100905. doi: 10.1016/j.resplu.2025.100905. eCollection 2025 Mar. Resusc Plus. 2025. PMID: 40084127 Free PMC article.
-
Effect of vasopressin on brain and cardiac tissue during neonatal cardiopulmonary resuscitation of asphyxiated post-transitional piglets.Resusc Plus. 2024 Dec 14;21:100837. doi: 10.1016/j.resplu.2024.100837. eCollection 2025 Jan. Resusc Plus. 2024. PMID: 39758757 Free PMC article.
References
-
- Wyckoff M.H., Wyllie J.P., Aziz K., de Almeida M.F., Fabres J., Fawke J., et al. Neonatal life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation. 2020;142:329–337. doi: 10.1161/cir.0000000000000895. - DOI - PubMed
-
- Aziz K., Lee H.C., Escobedo M.B., Hoover A.V., Kamath-Rayne B.D., Kapadia V.S., et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:1–27. doi: 10.1161/cir.0000000000000902. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical