Developing Versatile Contactors for Direct Air Capture of CO2 through Amine Grafting onto Alumina Pellets and Alumina Wash-Coated Monoliths
- PMID: 37663169
- PMCID: PMC10472440
- DOI: 10.1021/acs.iecr.3c01265
Developing Versatile Contactors for Direct Air Capture of CO2 through Amine Grafting onto Alumina Pellets and Alumina Wash-Coated Monoliths
Abstract
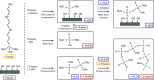
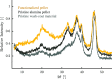
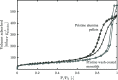
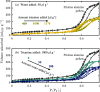
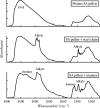
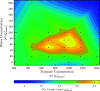
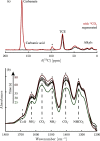
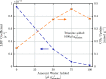
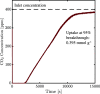
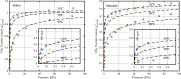
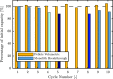
The optimization of the air-solid contactor is critical to improve the efficiency of the direct air capture (DAC) process. To enable comparison of contactors and therefore a step toward optimization, two contactors are prepared in the form of pellets and wash-coated honeycomb monoliths. The desired amine functionalities are successfully incorporated onto these industrially relevant pellets by means of a procedure developed for powders, providing materials with a CO2 uptake not influenced by the morphology and the structure of the materials according to the sorption measurements. Furthermore, the amine functionalities are incorporated onto alumina wash-coated monoliths that provide a similar CO2 uptake compared to the pellets. Using breakthrough measurements, dry CO2 uptakes of 0.44 and 0.4 mmol gsorbent-1 are measured for pellets and for a monolith, respectively. NMR and IR studies of CO2 uptake show that the CO2 adsorbs mainly in the form of ammonium carbamate. Both contactors are characterized by estimated Toth isotherm parameters and linear driving force (LDF) coefficients to enable an initial comparison and provide information for further studies of the two contactors. LDF coefficients of 1.5 × 10-4 and of 1.2 × 10-3 s-1 are estimated for the pellets and for a monolith, respectively. In comparison to the pellets, the monolith therefore exhibits particularly promising results in terms of adsorption kinetics due to its hierarchical pore structure. This is reflected in the productivity of the adsorption step of 6.48 mol m-3 h-1 for the pellets compared to 7.56 mol m-3 h-1 for the monolith at a pressure drop approximately 1 order of magnitude lower, making the monoliths prime candidates to enhance the efficiency of DAC processes.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
On Comparing Packed Beds and Monoliths for CO2 Capture from Air Through Experiments, Theory, and Modeling.Ind Eng Chem Res. 2024 Jun 18;63(26):11637-11653. doi: 10.1021/acs.iecr.4c01392. eCollection 2024 Jul 3. Ind Eng Chem Res. 2024. PMID: 38983186 Free PMC article.
-
Mass Transfer of CO2 in Amine-Functionalized Structured Contactors in Ultra-Dilute Conditions.Ind Eng Chem Res. 2025 Jan 13;64(4):2339-2353. doi: 10.1021/acs.iecr.4c04099. eCollection 2025 Jan 29. Ind Eng Chem Res. 2025. PMID: 39906288 Free PMC article.
-
Cold Temperature Direct Air CO2 Capture with Amine-Loaded Metal-Organic Framework Monoliths.ACS Appl Mater Interfaces. 2024 Jan 10;16(1):1404-1415. doi: 10.1021/acsami.3c13528. Epub 2023 Dec 18. ACS Appl Mater Interfaces. 2024. PMID: 38109480 Free PMC article.
-
Critical Comparison of Structured Contactors for Adsorption-Based Gas Separations.Annu Rev Chem Biomol Eng. 2018 Jun 7;9:129-152. doi: 10.1146/annurev-chembioeng-060817-084120. Epub 2018 Mar 26. Annu Rev Chem Biomol Eng. 2018. PMID: 29579401 Review.
-
Recent advances in direct air capture by adsorption.Chem Soc Rev. 2022 Aug 1;51(15):6574-6651. doi: 10.1039/d1cs00970b. Chem Soc Rev. 2022. PMID: 35815699 Review.
Cited by
-
Exploring Geometric Properties and Cycle Design in Packed Bed and Monolith Contactors Using Temperature-Vacuum Swing Adsorption Modeling for Direct Air Capture.Ind Eng Chem Res. 2024 Nov 4;63(45):19728-19743. doi: 10.1021/acs.iecr.4c02303. eCollection 2024 Nov 13. Ind Eng Chem Res. 2024. PMID: 39553914 Free PMC article.
-
Self-Supported Branched Poly(ethylenimine) Monoliths from Inverse Template 3D Printing for Direct Air Capture.ACS Appl Mater Interfaces. 2025 Feb 19;17(7):10696-10709. doi: 10.1021/acsami.4c20617. Epub 2025 Feb 11. ACS Appl Mater Interfaces. 2025. PMID: 39931906 Free PMC article.
-
Measuring and Modeling Water and Carbon Dioxide Adsorption on Amine Functionalized Alumina under Direct Air Capture Conditions.Ind Eng Chem Res. 2025 Mar 19;64(13):7165-7175. doi: 10.1021/acs.iecr.4c04581. eCollection 2025 Apr 2. Ind Eng Chem Res. 2025. PMID: 40191641 Free PMC article.
-
Contributions of CO2, O2, and H2O to the Oxidative Stability of Solid Amine Direct Air Capture Sorbents at Intermediate Temperature.ACS Appl Mater Interfaces. 2023 Oct 11;15(40):46790-46802. doi: 10.1021/acsami.3c08140. Epub 2023 Sep 29. ACS Appl Mater Interfaces. 2023. PMID: 37774150 Free PMC article.
-
On Comparing Packed Beds and Monoliths for CO2 Capture from Air Through Experiments, Theory, and Modeling.Ind Eng Chem Res. 2024 Jun 18;63(26):11637-11653. doi: 10.1021/acs.iecr.4c01392. eCollection 2024 Jul 3. Ind Eng Chem Res. 2024. PMID: 38983186 Free PMC article.
References
-
- Climate Change and International Responses Increasing Challenges to US National Security Through 2040; United States National Intelligence Council: Washingtion D.C., USA, 2021.
-
- Climate Change and Sustainability Strategic Approach; United Kingdom Ministry of Defence: London, U.K., 2021.
-
- IPCC, In Climate Change 2022: Mitigation of Climate Change. Contribution of Working Group III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Shukla P.; Skea J.; Slade R.; Khourdajie A. A.; van Diemen R.; McCollum D.; Pathak M.; Some S.; Vyas P.; Fradera R.; Belkacemi M.; Hasija A.; Lisboa G.; Luz S.; Malley J., Eds.; Cambridge University Press, 2022.
-
- IPCC, Global Warming of 1.5°C: IPCC Special Report on Impacts of Global Warming of 1.5°C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Cambridge University Press, 2018.
-
- Lackner K. S. The thermodynamics of direct air capture of carbon dioxide. Energy 2013, 50, 38–46. 10.1016/j.energy.2012.09.012. - DOI
LinkOut - more resources
Full Text Sources
