Dry Swab-Based Nucleic Acid Extraction vs. Spin Column-Based Nucleic Acid Extraction for COVID-19 RT-PCR Testing: A Comparative Study
- PMID: 37663452
- PMCID: PMC10469701
- DOI: 10.1155/2023/6624932
Dry Swab-Based Nucleic Acid Extraction vs. Spin Column-Based Nucleic Acid Extraction for COVID-19 RT-PCR Testing: A Comparative Study
Abstract
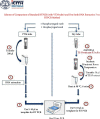
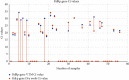
Conventional nucleic acid extraction involves usage of spin columns to isolate the RNA, but this is labor intensive. This study compares the spin column method with a dry swab-based method of extraction using a proteinase K buffer and subsequent heat inactivation. A total of 56 subjects were tested for COVID-19 by RT-PCR with probes targeting the E and RdRp genes by collecting two nasopharyngeal and two oropharyngeal swabs and subjecting one set to nucleic acid extraction by spin column and the other set to dry swab-based methods. Out of the 56 samples tested, 27 were positive for VTM-based extraction and 29 were negative. Dry swab-based extraction produced 22 positive results (sensitivity = 81.48%) and 34 negative results. The E gene was detectable in 25 samples by the dry swab method out of 27 samples that tested positive by the VTM-based method (sensitivity = 92.5%). The RdRp gene was detectable in 22 samples by the dry swab method out of 27 samples that tested positive by the VTM-based method (sensitivity = 81.48%). Concordance was 91% with discordance at 9% and a Kappa value of 0.82, indicating almost perfect agreement between the two methods. Our findings indicate that the dry swab method of nucleic acid extraction is a useful alternative to conventional spin column-based extraction with comparable sensitivity and specificity. The trial was registered with the Clinical Trials Registry of India (CTRI) with a CTRI registration number of CTRI/2021/12/038792.
Copyright © 2023 Mohammed Faraaz Khan and C. Roopa.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures




References
-
- World Health Organization. Technical Guidance. Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica... .
-
- World Health Organization. WHO coronavirus (COVID-19) dashboard. 2022. https://covid19.who.int/
-
- Chu A. W. H., Chan W. M., Ip J. D., Yip J. F. W., Yuen K.-Y. Evaluation of simple nucleic acid extraction methods for the detection of SARS-CoV-2 in nasopharyngeal and saliva specimens during global shortage of extraction kits. Journal of Clinical Virology . 2020;129 doi: 10.1016/j.jcv.2020.104519.104519 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

