Intravesical chemotherapy synergize with an immune adjuvant by a thermo-sensitive hydrogel system for bladder cancer
- PMID: 37663619
- PMCID: PMC10468327
- DOI: 10.1016/j.bioactmat.2023.08.013
Intravesical chemotherapy synergize with an immune adjuvant by a thermo-sensitive hydrogel system for bladder cancer
Abstract
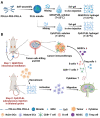
Surgical resection remains the prefer option for bladder cancer treatment. However, the effectiveness of surgery is usually limited for the high recurrence rate and poor prognosis. Consequently, intravesical chemotherapy synergize with immunotherapy in situ is an attractive way to improve therapeutic effect. Herein, a combined strategy based on thermo-sensitive PLEL hydrogel drug delivery system was developed. GEM loaded PLEL hydrogel was intravesical instilled to kill tumor cells directly, then PLEL hydrogel incorporated with CpG was injected into both groins subcutaneously to promote immune responses synergize with GEM. The results demonstrated that drug loaded PLEL hydrogel had a sol-gel phase transition behavior in response to physiological temperature and presented sustained drug release, and the PLEL-assisted combination therapy could have better tumor suppression effect and stronger immunostimulating effect in vivo. Hence, this combined treatment with PLEL hydrogel system has great potential and suggests a clinically-relevant and valuable option for bladder cancer.
Keywords: Bladder cancer; Immunotherapy; Intravesical chemotherapy; Localized drug delivery; Thermo-responsive hydrogel.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests in this paper.
Figures









Similar articles
-
Sustained co-delivery of 5-fluorouracil and cis-platinum via biodegradable thermo-sensitive hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer.Bioact Mater. 2022 Nov 8;23:1-15. doi: 10.1016/j.bioactmat.2022.10.004. eCollection 2023 May. Bioact Mater. 2022. PMID: 36406247 Free PMC article.
-
Cyclophosphamide loaded thermo-responsive hydrogel system synergize with a hydrogel cancer vaccine to amplify cancer immunotherapy in a prime-boost manner.Bioact Mater. 2021 Mar 9;6(10):3036-3048. doi: 10.1016/j.bioactmat.2021.03.003. eCollection 2021 Oct. Bioact Mater. 2021. PMID: 33778186 Free PMC article.
-
Sustained release of levobupivacaine from temperature-sensitive injectable hydrogel for long-term local anesthesia in postoperative pain management.Biomaterials. 2023 Aug;299:122129. doi: 10.1016/j.biomaterials.2023.122129. Epub 2023 May 1. Biomaterials. 2023. PMID: 37167892
-
Diagnosis and management of superficial bladder cancer.Curr Probl Cancer. 2001 Jul-Aug;25(4):219-78. doi: 10.1067/mcn.2001.117539. Curr Probl Cancer. 2001. PMID: 11514784 Review.
-
Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer.Urol Oncol. 2014 Jan;32(1):35.e21-30. doi: 10.1016/j.urolonc.2013.02.010. Epub 2013 Apr 28. Urol Oncol. 2014. PMID: 23628309 Review.
Cited by
-
Ferrous Selenide Stabilized Black Phosphorus Heterojunction Sonosensitizer for MR Imaging-Guided Sonodynamic Therapy of Bladder Cancer.Biomater Res. 2024 Mar 27;28:0014. doi: 10.34133/bmr.0014. eCollection 2024. Biomater Res. 2024. PMID: 38549610 Free PMC article.
-
Recent advances in innovative biomaterials for promoting bladder regeneration: processing and functionalization.Front Bioeng Biotechnol. 2025 Jan 6;12:1528658. doi: 10.3389/fbioe.2024.1528658. eCollection 2024. Front Bioeng Biotechnol. 2025. PMID: 39834643 Free PMC article. Review.
-
Strategies for intravesical drug delivery: From bladder physiological barriers and potential transport mechanisms.Acta Pharm Sin B. 2024 Nov;14(11):4738-4755. doi: 10.1016/j.apsb.2024.07.003. Epub 2024 Jul 6. Acta Pharm Sin B. 2024. PMID: 39664414 Free PMC article. Review.
-
Hydrogels as a Potential Biomaterial for Multimodal Therapeutic Applications.Mol Pharm. 2024 Oct 7;21(10):4827-4848. doi: 10.1021/acs.molpharmaceut.4c00595. Epub 2024 Sep 18. Mol Pharm. 2024. PMID: 39290162 Free PMC article. Review.
-
A KIF20A-based thermosensitive hydrogel vaccine effectively potentiates immune checkpoint blockade therapy for hepatocellular carcinoma.NPJ Vaccines. 2025 Jan 3;10(1):1. doi: 10.1038/s41541-024-01060-2. NPJ Vaccines. 2025. PMID: 39753573 Free PMC article.
References
-
- International Agency for Research on Cancer 2020. http://gco.iarc.fr/today/home.2020-04-05
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Kamat A.M., Hahn N.M., Efstathiou J.A., Lerner S.P., Malmstrom P.-U., Choi W., Guo C.C., Lotan Y., Kassouf W. Bladder cancer, Lancet. 2016;388:2796–2810. 10061. - PubMed
-
- Soria F., D'Andrea D., Abufaraj M., Moschini M., Giordano A., Gust K.M., Karakiewicz P.I., Babjuk M., Gontero P., Shariat S.F. Stratification of intermediate-risk non-muscle-invasive bladder cancer patients: implications for adjuvant therapies. Eur Urol Focus. 2021;7(3):566–573. - PubMed
-
- Lotan Y., Kamat A.M., Porter M.P., Robinson V.L., Shore N., Jewett M., Schelhammer P.F., White R.d., Quale D., Lee C.T. N. Bladder canc advocacy, O. Soc urologic, key concerns about the current state of bladder cancer A position paper from the bladder cancer think tank. Bladder Cancer Advocacy Netw. Soc. Urologic Oncol. Cancer. 2009;115(18):4096–4103. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

