Research advances of nanomaterials for the acceleration of fracture healing
- PMID: 37663621
- PMCID: PMC10474571
- DOI: 10.1016/j.bioactmat.2023.08.016
Research advances of nanomaterials for the acceleration of fracture healing
Abstract
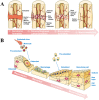
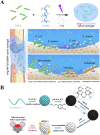
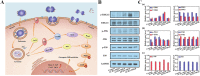
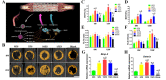
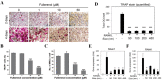
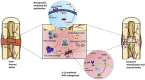
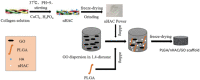
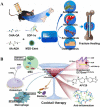
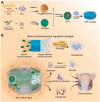
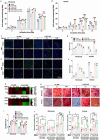
The bone fracture cases have been increasing yearly, accompanied by the increased number of patients experiencing non-union or delayed union after their bone fracture. Although clinical materials facilitate fracture healing (e.g., metallic and composite materials), they cannot fulfill the requirements due to the slow degradation rate, limited osteogenic activity, inadequate osseointegration ability, and suboptimal mechanical properties. Since early 2000, nanomaterials successfully mimic the nanoscale features of bones and offer unique properties, receiving extensive attention. This paper reviews the achievements of nanomaterials in treating bone fracture (e.g., the intrinsic properties of nanomaterials, nanomaterials for bone defect filling, and nanoscale drug delivery systems in treating fracture delayed union). Furthermore, we discuss the perspectives on the challenges and future directions of developing nanomaterials to accelerate fracture healing.
Keywords: Bone defect; Bone regeneration; Delayed union; Fracture healing; Nanomaterials; Non-union.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










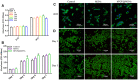
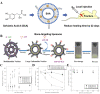


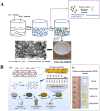
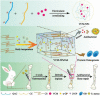
References
-
- Toosi S., Behravan N., Behravan J. Nonunion fractures, mesenchymal stem cells and bone tissue engineering. J. Biomed. Mater. Res., Part A. 2018;106:2552–2562. - PubMed
-
- Chen W., Lv H., Liu S., Liu B., Zhu Y., Chen X., Yang G., Liu L., Zhang T., Wang H., Yin B., Guo J., Zhang X., Li Y., Smith D., Hu P., Sun J., Zhang Y. National incidence of traumatic fractures in China: a retrospective survey of 512 187 individuals. Lancet Global Health. 2017;5:807–817. - PubMed
-
- Lv H., Chen W., Zhang T., Hou Z., Yang G., Zhu Y., Wang H., Yin B., Guo J., Liu L., Hu P., Liu S., Liu B., Sun J., Li S., Zhang X., Li Y., Zhang Y. Traumatic fractures in China from 2012 to 2014: a national survey of 512,187 individuals. Osteoporos. Int. 2020;31:2167–2178. - PubMed
-
- Buckwalter J.A., Brown T.D. Joint injury, repair, and remodeling. Clin. Orthop. Relat. Res. 2004;423:7–16. - PubMed
-
- Schuit S.C.E., van der Klift M., Weel A.E.A.M., de Laet C.E.D.H., Burger H., Seeman E., Hofman A., Uitterlinden A.G., van Leeuwen J.P.T.M., Pols H.A.P. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195–202. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

