Selection of viral capsids and promoters affects the efficacy of rescue of Tmprss3-deficient cochlea
- PMID: 37663645
- PMCID: PMC10471831
- DOI: 10.1016/j.omtm.2023.08.004
Selection of viral capsids and promoters affects the efficacy of rescue of Tmprss3-deficient cochlea
Abstract
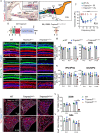
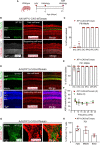
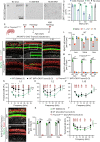
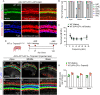
Adeno-associated virus (AAV)-mediated gene transfer has shown promise in rescuing mouse models of genetic hearing loss, but how viral capsid and promoter selection affects efficacy is poorly characterized. Here, we tested combinations of AAVs and promoters to deliver Tmprss3, mutations in which are associated with hearing loss in humans. Tmprss3tm1/tm1 mice display severe cochlear hair cell degeneration, loss of auditory brainstem responses, and delayed loss of spiral ganglion neurons. Under the ubiquitous CAG promoter and AAV-KP1 capsid, Tmprss3 overexpression caused striking cytotoxicity in vitro and in vivo and failed to rescue degeneration or dysfunction of the Tmprss3tm1/tm1 cochlea. Reducing the dosage or using AAV-DJ-CAG-Tmprss3 diminished cytotoxicity without rescue of the Tmprss3tm1/tm1 cochlea. Finally, the combination of AAV-KP1 capsid and the EF1α promoter prevented cytotoxicity and reduced hair cell degeneration, loss of spiral ganglion neurons, and improved hearing thresholds in Tmprss3tm1/tm1 mice. Together, our study illustrates toxicity of exogenous genes and factors governing rescue efficiency, and suggests that cochlear gene therapy likely requires precisely targeted transgene expression.
Keywords: Tmprss3; cochlea; gene therapy; hair cells; hearing loss; supporting cells.
© 2023 The Author(s).
Conflict of interest statement
K.P. and M.A.K. are inventors of filed patents held by Stanford University.
Figures

References
-
- Scott H.S., Kudoh J., Wattenhofer M., Shibuya K., Berry A., Chrast R., Guipponi M., Wang J., Kawasaki K., Asakawa S., et al. Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat. Genet. 2001;27:59–63. - PubMed
-
- Wattenhofer M., Sahin-Calapoglu N., Andreasen D., Kalay E., Caylan R., Braillard B., Fowler-Jaeger N., Reymond A., Rossier B.C., Karaguzel A., Antonarakis S.E. A novel TMPRSS3 missense mutation in a DFNB8/10 family prevents proteolytic activation of the protein. Hum. Genet. 2005;117:528–535. doi: 10.1007/s00439-005-1332-x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

