Mast cell-mediated immune regulation in health and disease
- PMID: 37663654
- PMCID: PMC10470157
- DOI: 10.3389/fmed.2023.1213320
Mast cell-mediated immune regulation in health and disease
Abstract
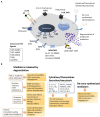
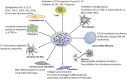
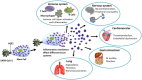
Mast cells are important components of the immune system, and they perform pro-inflammatory as well as anti-inflammatory roles in the complex process of immune regulation in health and disease. Because of their strategic perivascular localization, sensitivity and adaptability to the microenvironment, and ability to release a variety of preformed and newly synthesized effector molecules, mast cells perform unique functions in almost all organs. Additionally, Mast cells express a wide range of surface and cytoplasmic receptors which enable them to respond to a variety of cytokines, chemicals, and pathogens. The mast cell's role as a cellular interface between external and internal environments as well as between vasculature and tissues is critical for protection and repair. Mast cell interactions with different immune and nonimmune cells through secreted inflammatory mediators may also turn in favor of disease promoting agents. First and forefront, mast cells are well recognized for their multifaceted functions in allergic diseases. Reciprocal communication between mast cells and endothelial cells in the presence of bacterial toxins in chronic/sub-clinical infections induce persistent vascular inflammation. We have shown that mast cell proteases and histamine induce endothelial inflammatory responses that are synergistically amplified by bacterial toxins. Mast cells have been shown to exacerbate vascular changes in normal states as well as in chronic or subclinical infections, particularly among cigarette smokers. Furthermore, a potential role of mast cells in SARS-CoV-2-induced dysfunction of the capillary-alveolar interface adds to the growing understanding of mast cells in viral infections. The interaction between mast cells and microglial cells in the brain further highlights their significance in neuroinflammation. This review highlights the significant role of mast cells as the interface that acts as sensor and early responder through interactions with cells in systemic organs and the nervous system.
Keywords: SARS-CoV-2 disease; allergic disease; atherosclerosis; bacterial infection; cardiovascular disease; cigarette smoking; neuroinflammation; skin and wound-healing.
Copyright © 2023 Dileepan, Raveendran, Sharma, Abraham, Barua, Singh, Sharma and Sharma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Ehrlich P. Beitraege Zur Kenntniss Der Anilinfaerbungen Und Ihrer Verwendung in Der Mikroskopischen Technik. Arch mikr anat u entwcklngsmech. (1877) 13:263–77. doi: 10.1007/BF02933937 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

