Understanding the role of conserved proline and serine residues in the SARS-CoV-2 spike cleavage sites in the virus entry, fusion, and infectivity
- PMID: 37663753
- PMCID: PMC10469153
- DOI: 10.1007/s13205-023-03749-y
Understanding the role of conserved proline and serine residues in the SARS-CoV-2 spike cleavage sites in the virus entry, fusion, and infectivity
Abstract
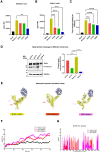
The spike (S) glycoprotein of the SARS-CoV-2 virus binds to the host cell receptor and promotes the virus's entry into the target host cell. This interaction is primed by host cell proteases like furin and TMPRSS2, which act at the S1/S2 and S2´ cleavage sites, respectively. Both cleavage sites have serine or proline residues flanking either the single or polybasic region and were found to be conserved in coronaviruses. Unravelling the effects of these conserved residues on the virus entry and infectivity might facilitate the development of novel therapeutics. Here, we have investigated the role of the conserved serine and proline residues in the SARS-CoV-2 spike mediated entry, fusogenicity, and viral infectivity by using the HIV-1/spike-based pseudovirus system. A conserved serine residue mutation to alanine (S2´S-A) at the S2´ cleavage site resulted in the complete loss of spike cleavage. Exogenous treatment with trypsin or overexpression of TMPRSS2 protease could not rescue the loss of spike cleavage and biological activity. The S2´S-A mutant showed no significant responses against E-64d, TMPRSS2 or other relevant inhibitors. Taken together, serine at the S2´ site in the spike protein was indispensable for spike protein cleavage and virus infectivity. Thus, novel interventions targeting the conserved serine at the S2´ cleavage site should be explored to reduce severe disease caused by SARS-CoV-2-and novel emerging variants.
Supplementary information: The online version contains supplementary material available at 10.1007/s13205-023-03749-y.
Keywords: Cell entry and viral infectivity; Conserved serine; SARS-CoV-2; Spike cleavage site.
© King Abdulaziz City for Science and Technology 2023. Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Conflict of interest statement
Conflict of interestOn behalf of all authors, the corresponding author states that there is no conflict of interest.
Figures



Similar articles
-
SARS-CoV-2 Spike Furin Cleavage Site and S2' Basic Residues Modulate the Entry Process in a Host Cell-Dependent Manner.J Virol. 2022 Jul 13;96(13):e0047422. doi: 10.1128/jvi.00474-22. Epub 2022 Jun 9. J Virol. 2022. PMID: 35678602 Free PMC article.
-
Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity.J Virol. 2022 Apr 27;96(8):e0012822. doi: 10.1128/jvi.00128-22. Epub 2022 Mar 28. J Virol. 2022. PMID: 35343766 Free PMC article.
-
Genome-wide bioinformatics analysis of human protease capacity for proteolytic cleavage of the SARS-CoV-2 spike glycoprotein.Microbiol Spectr. 2024 Feb 6;12(2):e0353023. doi: 10.1128/spectrum.03530-23. Epub 2024 Jan 8. Microbiol Spectr. 2024. PMID: 38189333 Free PMC article.
-
Roles of host proteases in the entry of SARS-CoV-2.Anim Dis. 2023;3(1):12. doi: 10.1186/s44149-023-00075-x. Epub 2023 Apr 25. Anim Dis. 2023. PMID: 37128508 Free PMC article. Review.
-
Proteolytic activation of SARS-CoV-2 spike protein.Microbiol Immunol. 2022 Jan;66(1):15-23. doi: 10.1111/1348-0421.12945. Epub 2021 Oct 12. Microbiol Immunol. 2022. PMID: 34561887 Free PMC article. Review.
Cited by
-
Innovations and Challenges in the Development of COVID-19 Vaccines for a Safer Tomorrow.Cureus. 2024 May 10;16(5):e60015. doi: 10.7759/cureus.60015. eCollection 2024 May. Cureus. 2024. PMID: 38854201 Free PMC article. Review.
-
Pseudotyped Viruses: A Useful Platform for Pre-Clinical Studies Conducted in a BSL-2 Laboratory Setting.Biomolecules. 2025 Jan 15;15(1):135. doi: 10.3390/biom15010135. Biomolecules. 2025. PMID: 39858529 Free PMC article. Review.
-
Mapping IgA Epitope and Cross-Reactivity between Severe Acute Respiratory Syndrome-Associated Coronavirus 2 and DENV.Vaccines (Basel). 2023 Nov 24;11(12):1749. doi: 10.3390/vaccines11121749. Vaccines (Basel). 2023. PMID: 38140154 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

