CuAAC-methacrylate interpenetrating polymer network (IPN) properties modulated by visible-light photoinitiation
- PMID: 37663952
- PMCID: PMC10470441
- DOI: 10.1039/d3py00507k
CuAAC-methacrylate interpenetrating polymer network (IPN) properties modulated by visible-light photoinitiation
Abstract
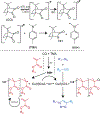
Interpenetrating polymer networks (IPNs) are a class of materials with interwoven polymers that exhibit unique blended or enhanced properties useful to a variety of applications, ranging from restorative protective materials to conductive membranes and hydrophobic adhesives. The IPN formation kinetics can play a critical role in the development of the underlying morphology and in turn the properties of the material. Dual photoinitiation of copper-catalyzed azide-alkyne (CuAAC) and radical mediated methacrylate polymerization chemistries enable the manipulation of IPN microstructure and properties by controlling the kinetics of IPN formation via the intensity of the initiating light. Specifically, azide and alkyne-based polyethylene glycol monomers and tetraethylene glycol dimethacrylate (TEGDMA) were polymerized in a single pot to form IPNs and the properties were evaluated as a function of the photoinitiating light intensity. Morphological differences as a function of intensity were observed in the IPNs as determined by thermomechanical properties and phase-contrast imaging in tapping mode atomic force microscopy (AFM). At moderate intensities (20 mW/cm2) of visible light (470 nm), the TEGDMA polymerization gels first and therefore forms the underlying network scaffold. At low intensities (0.2 mW/cm2), the CuAAC polymerization can gel first. The ability to switch sequence of gelation and IPN trajectory (simultaneous vs. sequential), affords control over phase separation behavior. Thus, light not only allows for spatial and temporal control over the IPN formation but also provides control over their thermomechanical properties, representing a route for facile IPNs design, synthesis, and application.
Conflict of interest statement
Conflicts of interest There are no conflicts to declare.
Figures





Similar articles
-
Evaluation of a photo-initiated copper(I)-catalyzed azide-alkyne cycloaddition polymer network with improved water stability and high mechanical performance as an ester-free dental restorative.Dent Mater. 2021 Oct;37(10):1592-1600. doi: 10.1016/j.dental.2021.08.010. Epub 2021 Aug 26. Dent Mater. 2021. PMID: 34456051
-
Tough, Transparent, Photocurable Hybrid Elastomers.ACS Appl Mater Interfaces. 2020 Sep 30;12(39):44125-44136. doi: 10.1021/acsami.0c11643. Epub 2020 Sep 17. ACS Appl Mater Interfaces. 2020. PMID: 32856894
-
Kinetics of bulk photo-initiated copper(i)-catalyzed azide-alkyne cycloaddition (CuAAC) polymerizations.Polym Chem. 2016 Jan 21;7(3):603-612. doi: 10.1039/c5py01655j. Epub 2015 Nov 18. Polym Chem. 2016. PMID: 27429650 Free PMC article.
-
Towards superior biopolymer gels by enabling interpenetrating network structures: A review on types, applications, and gelation strategies.Adv Colloid Interface Sci. 2024 Mar;325:103113. doi: 10.1016/j.cis.2024.103113. Epub 2024 Feb 15. Adv Colloid Interface Sci. 2024. PMID: 38387158 Review.
-
Characterization Methods to Determine Interpenetrating Polymer Network (IPN) in Hydrogels.Polymers (Basel). 2024 Jul 18;16(14):2050. doi: 10.3390/polym16142050. Polymers (Basel). 2024. PMID: 39065367 Free PMC article. Review.
References
-
- Gu Y, Zhao J and Johnson JA, Angewandte Chemie International Edition, 2020, 59, 5022–5049. - PubMed
-
- Sperling LH and Mishra V, Polymers for Advanced Technologies, 1996, 7, 197–208.
-
- Silverstein MS, Polymer, 2020, 207.
-
- Lipatov YS and Alekseeva TT, Phase-Separated Interpenetrating Polymer Networks, 2007.
-
- Anahidzade N, Dinari M, Abdolmaleki A, Tadavani KF and Zhiani M, Energy & Fuels, 2019, 33, 5749–5760.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
