Impact of the time interval between primary or interval surgery and adjuvant chemotherapy in ovarian cancer patients
- PMID: 37664032
- PMCID: PMC10468566
- DOI: 10.3389/fonc.2023.1221096
Impact of the time interval between primary or interval surgery and adjuvant chemotherapy in ovarian cancer patients
Abstract
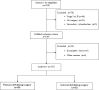
Introduction: Primary debulking surgery (PDS), interval debulking surgery (IDS), and platinum-based chemotherapy are the current standard treatments for advanced ovarian cancer (OC). The time to initiation of adjuvant chemotherapy (TTC) could influence patient outcomes.
Methods: We conducted a multicenter retrospective cohort study of advanced (International Federation of Gynecology and Obstetrics (FIGO) stage III or IV) OC treated between 2014 and 2018 to assess progression-free survival (PFS) and overall survival (OS) in relation to TTC. All patients underwent a germline multigene panel for BRCA1/2 evaluation.
Results: Among the 83 patients who underwent PDS, a TTC ≥ 60 days was associated with a shorter PFS (hazard ratio (HR) 2.02, 95% confidence interval (CI) 1.04-3.93, p = 0.038), although this association lost statistical significance when adjusting for residual disease (HR 1.52, 95% CI 0.75-3.06, p = 0.244, for TTC and HR 2.73, 95% CI 1.50-4.96, p = 0.001, for residual disease). Among 52 IDS patients, we found no evidence of an association between TTC and clinical outcomes. Ascites, type of chemotherapy, or germline BRCA1/2 mutational status did not influence TTC and were not associated with clinical outcomes in PDS or IDS patients.
Discussion: In conclusion, longer TTC seems to negatively affect prognosis in patients undergoing PDS, especially those with residual disease.
Keywords: BRCA1/2 mutation; interval debulking surgery; ovarian cancer prognosis; primary debulking surgery; residual disease; time to initiation of chemotherapy.
Copyright © 2023 Farolfi, Petracci, Gurioli, Tedaldi, Casanova, Arcangeli, Amadori, Rosati, Stefanetti, Burgio, Cursano, Lolli, Zampiga, Cangini, Schepisi and Giorgi.
Conflict of interest statement
UG has received advisory board or consultancy fees from Merck Sharp & Dohme, Bristol Myers Squibb, Janssen, Astellas, Sanofi, Bayer, Pfizer, Ipsen, Novartis, and Pharmamar and institutional research grants from AstraZeneca, Sanofi, and Roche. AF has received personal honoraria for lectures from AstraZeneca, GSK-Tesaro, and Clovis and is on the advisory board of Janssen, AstraZeneca, and GSK-Tesaro. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Colombo N, Ledermann JA, ESMO Guidelines Committee . Electronic address: clinicalguidelines@esmo.org. Updated treatment recommendations for newly diagnosed epithelial ovarian carcinoma from the ESMO Clinical Practice Guidelines. Ann Oncol (2021) 32:1300–3. doi: 10.1016/j.annonc.2021.07.004 - DOI - PubMed
-
- Fagotti A, Ferrandina MG, Vizzielli G, Pasciuto T, Fanfani F, Gallotta V, et al. Randomized trial of primary debulking surgery versus neoadjuvant chemotherapy for advanced epithelial ovarian cancer (SCORPION-NCT01461850). Int J Gynecol Cancer (2020) 30:1657–64. - PubMed
-
- Colombo N, Sessa C, Bois AD, Ledermann J, McCluggage WG, McNeish I, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer (2019) 2019:ijgc–2019-000308. doi: 10.1136/ijgc-2019-000308 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

