Baseline whole-lung CT features deriving from deep learning and radiomics: prediction of benign and malignant pulmonary ground-glass nodules
- PMID: 37664069
- PMCID: PMC10470826
- DOI: 10.3389/fonc.2023.1255007
Baseline whole-lung CT features deriving from deep learning and radiomics: prediction of benign and malignant pulmonary ground-glass nodules
Abstract
Objective: To develop and validate the model for predicting benign and malignant ground-glass nodules (GGNs) based on the whole-lung baseline CT features deriving from deep learning and radiomics.
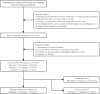
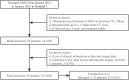
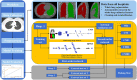
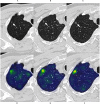
Methods: This retrospective study included 385 GGNs from 3 hospitals, confirmed by pathology. We used 239 GGNs from Hospital 1 as the training and internal validation set; 115 and 31 GGNs from Hospital 2 and Hospital 3 as the external test sets 1 and 2, respectively. An additional 32 stable GGNs from Hospital 3 with more than five years of follow-up were used as the external test set 3. We evaluated clinical and morphological features of GGNs at baseline chest CT and extracted the whole-lung radiomics features simultaneously. Besides, baseline whole-lung CT image features are further assisted and extracted using the convolutional neural network. We used the back-propagation neural network to construct five prediction models based on different collocations of the features used for training. The area under the receiver operator characteristic curve (AUC) was used to compare the prediction performance among the five models. The Delong test was used to compare the differences in AUC between models pairwise.
Results: The model integrated clinical-morphological features, whole-lung radiomic features, and whole-lung image features (CMRI) performed best among the five models, and achieved the highest AUC in the internal validation set, external test set 1, and external test set 2, which were 0.886 (95% CI: 0.841-0.921), 0.830 (95%CI: 0.749-0.893) and 0.879 (95%CI: 0.712-0.968), respectively. In the above three sets, the differences in AUC between the CMRI model and other models were significant (all P < 0.05). Moreover, the accuracy of the CMRI model in the external test set 3 was 96.88%.
Conclusion: The baseline whole-lung CT features were feasible to predict the benign and malignant of GGNs, which is helpful for more refined management of GGNs.
Keywords: X-ray computed; deep learning; ground-glass nodules; lung cancer; radiomics; tomography.
Copyright © 2023 Huang, Deng, Li, Xiong, Zhou, Ge, Zhang, Jing, Geng, Wang, Tu, Dong, Liu and Fan.
Conflict of interest statement
Author ZX was employed by Tron technology. Author YYG was employed by Shukun Beijing Technology Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

