Differential Diagnosis of Tumor-like Brain Lesions
- PMID: 37664132
- PMCID: PMC10468256
- DOI: 10.1212/CPJ.0000000000200182
Differential Diagnosis of Tumor-like Brain Lesions
Abstract
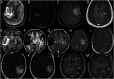
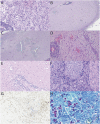
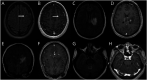
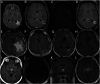
Purpose of review: Tumor-like brain lesions are rare and commonly suggest a neoplastic etiology. Failure to rapidly identify non-neoplastic causes can lead to increased morbidity and mortality. In this review, we describe 10 patients who presented with atypical, non-neoplastic tumor-like brain lesions in which brain biopsy was essential for a correct diagnosis and treatment.
Recent findings: There has been increasing recognition of autoimmune conditions affecting the nervous system, and many of those diseases can cause tumor-like brain lesions. Currently available reports of non-neoplastic tumor-like brain lesions are scarce. Most case series focus on tumefactive demyelinating lesions, and a comprehensive review including other neuroimmunological conditions such as CNS vasculitis, neurosarcoidosis, histiocytic and infectious etiologies is lacking.
Summary: We review the literature on tumor-like brain lesions intending to increase the awareness and differential diagnosis of non-neoplastic brain tumor mimics. We advocate for earlier brain biopsies, which, in our case series, significantly changed diagnosis, management, and outcomes.
© 2023 American Academy of Neurology.
Conflict of interest statement
G.S. Perez reports no disclosures relevant to the manuscript; L. Singer reports no disclosures relevant to the manuscript; T. Cao reports no disclosures relevant to the manuscript; P. Jamshidi reports no disclosures relevant to the manuscript; K. Dixit reports no disclosures relevant to the manuscript; M. Kontzialis reports no disclosures relevant to the manuscript; R. Castellani reports no disclosures relevant to the manuscript; P. Pytel reports no disclosures relevant to the manuscript; N. Anadani reports no disclosures relevant to the manuscript; C. Bevan has participated in an ad board for Genentech; E. Grebenciucova reports no disclosures relevant to the manuscript; R. Balabanov received honoraria from Biogen, Sanofi, Alexion, and Teva Pharmaceutical and research support from the National Multiple Sclerosis Society, National Institute of Health, Nextcure, and Biogen; B. Cohen reports no disclosures relevant to the manuscript. E. Graham received consulting and advisory board fees from Novartis, Atara Biotherapeutics, Tavistock Life Sciences, Horizon Therapeutics, Roche Genentech. She receives research support from F. Hoffman-La Roche Ltd. She received compensation for question writing from ACP MKSAP. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
