Ion channels in cancer-induced bone pain: from molecular mechanisms to clinical applications
- PMID: 37664239
- PMCID: PMC10469682
- DOI: 10.3389/fnmol.2023.1239599
Ion channels in cancer-induced bone pain: from molecular mechanisms to clinical applications
Abstract
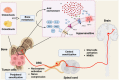
Cancer-induced bone pain (CIBP) caused by bone metastasis is one of the most prevalent diseases, and current treatments rely primarily on opioids, which have significant side effects. However, recent developments in pharmaceutical science have identified several new mechanisms for CIBP, including the targeted modification of certain ion channels and receptors. Ion channels are transmembrane proteins, which are situated on biological cell membranes, which facilitate passive transport of inorganic ions across membranes. They are involved in various physiological processes, including transmission of pain signals in the nervous system. In recent years, there has been an increasing interest in the role of ion channels in chronic pain, including CIBP. Therefore, in this review, we summarize the current literature on ion channels, related receptors, and drugs and explore the mechanism of CIBP. Targeting ion channels and regulating their activity might be key to treating pain associated with bone cancer and offer new treatment avenues.
Keywords: antinociception; cancer-induced bone pain; chronic pain; clinical application; ion channels.
Copyright © 2023 Lu, Wu and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

