Biglycan regulated colorectal cancer progress by modulating enteric neuron-derived IL-10 and abundance of Bacteroides thetaiotaomicron
- PMID: 37664615
- PMCID: PMC10469580
- DOI: 10.1016/j.isci.2023.107515
Biglycan regulated colorectal cancer progress by modulating enteric neuron-derived IL-10 and abundance of Bacteroides thetaiotaomicron
Abstract
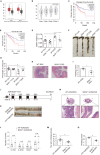
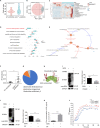
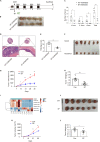
Biglycan (BGN) is a proteoglycan with branch chains and highly expressed in enteric neurons in the tumor tissue of colorectal cancer (CRC), which is negatively associated with survival rates in patients with CRC. However, how the proteoglycan promotes the progress of CRC through interacting with bacteria and regulating the immune response of enteric neurons remains largely unknown. In the present study, we found that biglycan deficiency changed tumor distribution in a colitis-associated colon cancer model. Furthermore, we revealed that BGN deficiency inhibits tumor growth in an allograft tumor model and the migration of cancer cell by upregulating interleukin-10 expression in enteric neurons. Significantly, we demonstrated that biglycan deficiency enriched the abundance of Bacteroides thetaiotaomicron through competing with it for chondroitin sulfate to inhibit CRC progress. Our work provided new insights into the interaction between host proteoglycan and gut microbiota as well as the role of enteric neurons in the tumor microenvironment.
Keywords: Cancer; Health sciences; Microbiome.
© 2023 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kvorjak M., Ahmed Y., Miller M.L., Sriram R., Coronnello C., Hashash J.G., Hartman D.J., Telmer C.A., Miskov-Zivanov N., Finn O.J., Cascio S. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2020;8:167–178. doi: 10.1158/2326-6066.CIR-19-0514. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

