Molecular biomarkers for facilitating genome‑directed precision medicine in gynecological cancer (Review)
- PMID: 37664647
- PMCID: PMC10472042
- DOI: 10.3892/ol.2023.14012
Molecular biomarkers for facilitating genome‑directed precision medicine in gynecological cancer (Review)
Abstract
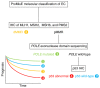
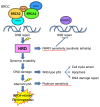
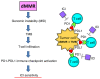
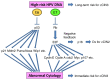
Prominent recent advancements in cancer treatment include the development and clinical application of next-generation sequencing (NGS) technologies, alongside a diverse array of novel molecular targeting therapeutics. NGS has enabled the high-speed and low-cost sequencing of whole genomes in individual patients, which has opened the era of genome-based precision medicine. The development of numerous molecular targeting agents, including anti-VEGF antibodies, poly (ADP-ribose) polymerase inhibitors and immune checkpoint inhibitors, have all improved the efficacy of systemic cancer therapy. Accumulating bench and translational research evidence has led to identification of various cancer-related biomarker profiles. In particular, companion diagnostics have been developed for some of these biomarkers, which can be clinically applied and are now widely used for guiding cancer therapies. Selecting biomarkers accurately will improve therapeutic efficacy, avoid overtreatment, enable earlier diagnosis and reduce the cost of preventing and treating gynecological cancer. Therefore, biomarkers are fast becoming indispensable tools in the practice of genome-directed precision medicine. In the present review, the current evidence of cancer-related biomarkers in the field of gynecological oncology, their molecular interpretations and future perspectives are outlined. The aim of the present review is to provide potentially useful information for the formulation of clinical trials.
Keywords: PARP inhibitor; biomarker; companion diagnostic; gynecological cancer; immune checkpoint inhibitor.
Copyright: © Minaguchi et al.
Conflict of interest statement
TS received participant/speaker/advisor/chair payments from Aska Pharmaceutical, AstraZeneca, Bayer Yakuhin, Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Fuji Pharma, GE HealthCare, Johnson & Johnson, Kyowa Kirin, Merck, Mochida Pharmaceutical, Nippon Kayaku, Nobelpharma, Otsuka Pharmaceutical, Pfizer, Taiho Pharmaceutical, Takeda Pharmaceutical, Tsumura and Yakult Honsha. AS received speaker/chair payments from AstraZeneca, Eisai, Johnson & Johnson, Medtronic, Merck, Sanofi S.A., Taiho Pharmaceutical and Takeda Pharmaceutical. AA received speaker payments from MSD and Takeda Pharmaceutical.
Figures
References
-
- Herzog TJ, Vergote I, Gomella LG, Milenkova T, French T, Tonikian R, Poehlein C, Hussain M. Testing for homologous recombination repair or homologous recombination deficiency for poly (ADP-ribose) polymerase inhibitors: A current perspective. Eur J Cancer. 2023;179:136–146. doi: 10.1016/j.ejca.2022.10.021. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
