Improved survival of SARS COV-2-infected K18- hACE2 mice treated with adenosine A2AR agonist
- PMID: 37664715
- PMCID: PMC10469936
- DOI: 10.1016/j.heliyon.2023.e19226
Improved survival of SARS COV-2-infected K18- hACE2 mice treated with adenosine A2AR agonist
Abstract
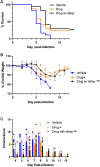
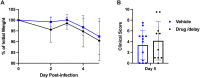
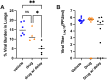
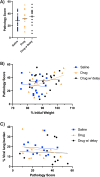
A life-threatening manifestation of Covid-19 infection is a cytokine storm that requires hospitalization and supplemental oxygen. Various strategies to reduce inflammatory cytokines have had some success in limiting cytokine storm and improving survival. Agonists of adenosine A2A receptors (A2AR) reduce cytokine release from most immune cells. Apadenoson is a potent and selective anti-inflammatory adenosine analog that reduces inflammation. When administered by subcutaneous osmotic pumps to mice infected with SARS CoV-2, Apadenoson was found to improve the outcomes of infection as measured by a decrease in weight loss, improved clinical symptoms, reduced levels of proinflammatory cytokines and chemokines in bronchial lavage (BAL) fluid, and enhanced survival of K18-hACE2 transgenic mice. These results support further examination of A2AR agonists as therapies for treating cytokine storm due to COVID-19.
Keywords: Adenosine agonists; Apadenoson; COVID-19; SARS CoV2; Therapy.
© 2023 The Authors.
Conflict of interest statement
PC, JL, BJM, and KLB have a patent pending on the use of A2AR agonists for the treatment of COVIID-19. The other authors declare no conflict of interest.The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.
Figures




Similar articles
-
Regadenoson for the treatment of COVID-19: A five case clinical series and mouse studies.PLoS One. 2023 Aug 11;18(8):e0288920. doi: 10.1371/journal.pone.0288920. eCollection 2023. PLoS One. 2023. PMID: 37566593 Free PMC article.
-
Animal Models of COVID-19: Transgenic Mouse Model.Methods Mol Biol. 2022;2452:259-289. doi: 10.1007/978-1-0716-2111-0_16. Methods Mol Biol. 2022. PMID: 35554912 Free PMC article.
-
Infectious Clones Produce SARS-CoV-2 That Causes Severe Pulmonary Disease in Infected K18-Human ACE2 Mice.mBio. 2021 Apr 20;12(2):e00819-21. doi: 10.1128/mBio.00819-21. mBio. 2021. PMID: 33879586 Free PMC article.
-
K18- and CAG-hACE2 Transgenic Mouse Models and SARS-CoV-2: Implications for Neurodegeneration Research.Molecules. 2022 Jun 28;27(13):4142. doi: 10.3390/molecules27134142. Molecules. 2022. PMID: 35807384 Free PMC article. Review.
-
Targeting Specific Checkpoints in the Management of SARS-CoV-2 Induced Cytokine Storm.Life (Basel). 2022 Mar 25;12(4):478. doi: 10.3390/life12040478. Life (Basel). 2022. PMID: 35454970 Free PMC article. Review.
Cited by
-
Adenosine A2AR in viral immune evasion and therapy: unveiling new avenues for treating COVID-19 and AIDS.Mol Biol Rep. 2024 Aug 8;51(1):894. doi: 10.1007/s11033-024-09839-1. Mol Biol Rep. 2024. PMID: 39115571 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

