Glucose dysregulation promotes oncogenesis in human bladder cancer by regulating autophagy and YAP1/TAZ expression
- PMID: 37665055
- PMCID: PMC10718143
- DOI: 10.1111/jcmm.17943
Glucose dysregulation promotes oncogenesis in human bladder cancer by regulating autophagy and YAP1/TAZ expression
Abstract
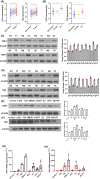
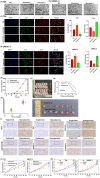
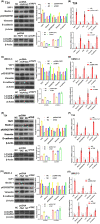
Glucose dysregulation is strongly correlated with cancer development, and cancer is prevalent in patients with Type 2 diabetes (T2D). We aimed to elucidate the mechanism underlying autophagy in response to glucose dysregulation in human bladder cancer (BC). 220 BC patients were included in this retrospective study. The expression of YAP1, TAZ and AMPK, EMT-associated markers, and autophagy marker proteins was analysed by immunohistochemistry, western blotting, and quantitative real-time PCR (qPCR). Further, T24 and UMUC-3 BC cells were cultured in media with different glucose concentrations, and the expression of YAP1, TAZ, AMPK and EMT-associated markers, and autophagy marker proteins was analysed by western blotting and qPCR. Autophagy was observed by immunofluorescence and electron microscopy. BC cell viability was tested using MTT assays. A xenograft nude mouse model of diabetes was used to evaluate tumour growth, metastasis and survival. A poorer pathologic grade and tumour-node-metastasis stage were observed in patients with BC with comorbid T2D than in others with BC. YAP1 and TAZ were upregulated in BC samples from patients with T2D. Mechanistically, high glucose (HG) promoted BC progression both in vitro and in vivo and inhibited autophagy. Specifically, various autophagy marker proteins and AMPK were negatively regulated under HG conditions and correlated with YAP1 and TAZ expression. These results demonstrate that HG inhibits autophagy and promotes cancer development in BC. YAP1/TAZ/AMPK signalling plays a crucial role in regulating glucose dysregulation during autophagy. Targeting these effectors exhibits therapeutic significance and can serve as prognostic markers in BC patients with T2D.
Keywords: AMPK; YAP1/TAZ; autophagy; bladder cancer; high glucose.
© 2023 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Glucose promotes epithelial-mesenchymal transitions in bladder cancer by regulating the functions of YAP1 and TAZ.J Cell Mol Med. 2020 Sep;24(18):10391-10401. doi: 10.1111/jcmm.15653. Epub 2020 Jul 17. J Cell Mol Med. 2020. PMID: 32678516 Free PMC article.
-
BET inhibitor JQ1 suppresses cell proliferation via inducing autophagy and activating LKB1/AMPK in bladder cancer cells.Cancer Med. 2019 Aug;8(10):4792-4805. doi: 10.1002/cam4.2385. Epub 2019 Jun 28. Cancer Med. 2019. PMID: 31250978 Free PMC article.
-
High Glucose Accelerates Tumor Progression by Regulating MEDAG-Mediated Autophagy Levels in Breast Cancer.Int J Biol Sci. 2022 Jul 4;18(11):4289-4300. doi: 10.7150/ijbs.70002. eCollection 2022. Int J Biol Sci. 2022. PMID: 35864962 Free PMC article.
-
Emerging role of Hippo signalling pathway in bladder cancer.J Cell Mol Med. 2018 Jan;22(1):4-15. doi: 10.1111/jcmm.13293. Epub 2017 Aug 7. J Cell Mol Med. 2018. PMID: 28782275 Free PMC article. Review.
-
Targeting YAP1/TAZ in nonsmall-cell lung carcinoma: From molecular mechanisms to precision medicine.Int J Cancer. 2023 Feb 15;152(4):558-571. doi: 10.1002/ijc.34249. Epub 2022 Aug 29. Int J Cancer. 2023. PMID: 35983734 Review.
Cited by
-
The Impact of Diabetes and Antidiabetics on Uro-Oncological Disease Outcomes: A Single-Center Experience.Urol Int. 2025;109(4):361-371. doi: 10.1159/000543757. Epub 2025 Jan 24. Urol Int. 2025. PMID: 39864425 Free PMC article.
-
circELP2 reverse-splicing biogenesis and function as a pro-fibrogenic factor by targeting mitochondrial quality control pathway.J Cell Mol Med. 2024 Feb;28(3):e18098. doi: 10.1111/jcmm.18098. Epub 2023 Dec 30. J Cell Mol Med. 2024. PMID: 38159063 Free PMC article.
-
A mendelian randomization study on the association between type 2 diabetes and the risk of bladder cancer.Discov Oncol. 2025 Apr 2;16(1):446. doi: 10.1007/s12672-025-02218-7. Discov Oncol. 2025. PMID: 40172743 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- 202108330204/China Scholarship Council
- 2022E10022/Key Laboratory of Clinical Laboratory Diagnosis and Translational Research of Zhejiang Province
- 81902555/National Natural Science Foundation of China
- Y2020150/Wenzhou Municipal Science and Technology Bureau
- QCXM202017/Youth Science and Technology Talent Innovation Project of Hainan Provincial Association for Science and Technology
LinkOut - more resources
Full Text Sources
Medical

