Id2 GABAergic interneurons comprise a neglected fourth major group of cortical inhibitory cells
- PMID: 37665123
- PMCID: PMC10581691
- DOI: 10.7554/eLife.85893
Id2 GABAergic interneurons comprise a neglected fourth major group of cortical inhibitory cells
Abstract
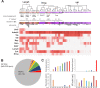
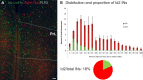
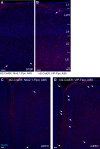
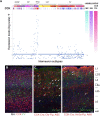
Cortical GABAergic interneurons (INs) represent a diverse population of mainly locally projecting cells that provide specialized forms of inhibition to pyramidal neurons and other INs. Most recent work on INs has focused on subtypes distinguished by expression of Parvalbumin (PV), Somatostatin (SST), or Vasoactive Intestinal Peptide (VIP). However, a fourth group that includes neurogliaform cells (NGFCs) has been less well characterized due to a lack of genetic tools. Here, we show that these INs can be accessed experimentally using intersectional genetics with the gene Id2. We find that outside of layer 1 (L1), the majority of Id2 INs are NGFCs that express high levels of neuropeptide Y (NPY) and exhibit a late-spiking firing pattern, with extensive local connectivity. While much sparser, non-NGFC Id2 INs had more variable properties, with most cells corresponding to a diverse group of INs that strongly expresses the neuropeptide CCK. In vivo, using silicon probe recordings, we observed several distinguishing aspects of NGFC activity, including a strong rebound in activity immediately following the cortical down state during NREM sleep. Our study provides insights into IN diversity and NGFC distribution and properties, and outlines an intersectional genetics approach for further study of this underappreciated group of INs.
Keywords: GABAergic Interneurons; Id2; cortical inhibition; in vivo recordings; intersectional genetics; mouse; neurogliaform cells; neuroscience.
© 2023, Machold et al.
Conflict of interest statement
RM, SD, MV, HZ, IK, JM, JH, YH, BS, GB, BR No competing interests declared
Figures





Update of
- doi: 10.1101/2022.12.01.518752
References
-
- Allen Institute for Brain Science Transcriptomics Explorer. 2023. [August 4, 2023]. https://celltypes.brain-map.org/rnaseq/mouse_ctx-hpf_10x
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

