Clinical Usefulness of a Cell-based Assay for Detecting Myelin Oligodendrocyte Glycoprotein Antibodies in Central Nervous System Inflammatory Disorders
- PMID: 37665286
- PMCID: PMC10485852
- DOI: 10.3343/alm.2024.44.1.56
Clinical Usefulness of a Cell-based Assay for Detecting Myelin Oligodendrocyte Glycoprotein Antibodies in Central Nervous System Inflammatory Disorders
Abstract
Background: The clinical implications of myelin oligodendrocyte glycoprotein autoantibodies (MOG-Abs) are increasing. Establishing MOG-Ab assays is essential for effectively treating patients with MOG-Abs. We established an in-house cell-based assay (CBA) to detect MOG-Abs to identify correlations with patients' clinical characteristics.
Methods: We established the CBA using HEK 293 cells transiently overexpressing full-length human MOG, tested it against 166 samples from a multicenter registry of central nervous system (CNS) inflammatory disorders, and compared the results with those of the Oxford MOG-Ab-based CBA and a commercial MOG-Ab CBA kit. We recruited additional patients with MOG-Abs and compared the clinical characteristics of MOG-Ab-associated disease (MOGAD) with those of neuromyelitis optica spectrum disorder (NMOSD).
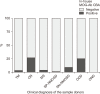
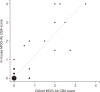
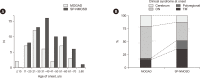
Results: Of 166 samples tested, 10 tested positive for MOG-Abs, with optic neuritis (ON) being the most common manifestation (4/15, 26.7%). The in-house and Oxford MOG-Ab CBAs agreed for 164/166 (98.8%) samples (κ=0.883, P<0.001); two patients (2/166, 1.2%) were only positive in our in-house CBA, and the CBA scores of the two laboratories correlated well (r=0.663, P<0.001). The commercial MOG-Ab CBA kit showed one false-negative and three false-positive results. The clinical presentation at disease onset differed between MOGAD and NMOSD; ON was the most frequent manifestation in MOGAD, and transverse myelitis was most frequent in NMOSD.
Conclusions: The in-house CBA for MOG-Abs demonstrated reliable results and can potentially be used to evaluate CNS inflammatory disorders. A comprehensive, long-term study with a large patient population would clarify the clinical significance of MOG-Abs.
Keywords: Autoantibody; Central nervous system disease; Immunoassay; Myelin oligodendrocyte glycoprotein.
Conflict of interest statement
Min J-H is funded by and has received research support from the National Research Foundation of Korea and an SMC Research and Development Grant. She has lectured for, consulted with, and received honoraria from Bayer Schering Pharma, Merck, Biogen Idec, Sanofi, UCB, Samsung Bioepis, Mitsubishi Tanabe, Celltrion, Roche, and Janssen. All other authors report no relevant disclosures.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

