The skin barrier: An extraordinary interface with an exceptional lipid organization
- PMID: 37666282
- PMCID: PMC10841493
- DOI: 10.1016/j.plipres.2023.101252
The skin barrier: An extraordinary interface with an exceptional lipid organization
Abstract
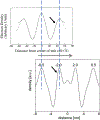
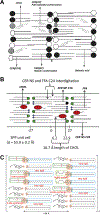
The barrier function of the skin is primarily located in the stratum corneum (SC), the outermost layer of the skin. The SC is composed of dead cells with highly organized lipid lamellae in the intercellular space. As the lipid matrix forms the only continuous pathway, the lipids play an important role in the permeation of compounds through the SC. The main lipid classes are ceramides (CERs), cholesterol (CHOL) and free fatty acids (FFAs). Analysis of the SC lipid matrix is of crucial importance in understanding the skin barrier function, not only in healthy skin, but also in inflammatory skin diseases with an impaired skin barrier. In this review we provide i) a historical overview of the steps undertaken to obtain information on the lipid composition and organization in SC of healthy skin and inflammatory skin diseases, ii) information on the role CERs, CHOL and FFAs play in the lipid phase behavior of very complex lipid model systems and how this knowledge can be used to understand the deviation in lipid phase behavior in inflammatory skin diseases, iii) knowledge on the role of both, CER subclasses and chain length distribution, on lipid organization and lipid membrane permeability in complex and simple model systems with synthetic CERs, CHOL and FFAs, iv) similarity in lipid phase behavior in SC of different species and complex model systems, and vi) future directions in modulating lipid composition that is expected to improve the skin barrier in inflammatory skin diseases.
Keywords: Inflammatory skin diseases; Permeability; Skin barrier function; Stratum corneum lipid composition; Stratum corneum lipids.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no competing financial interest.
Figures








References
-
- Wertz PW. Epidermal Lipids. Seminars in Dermatology 1992;11(2):106–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

