Assay-agnostic spatial profiling detects tumor microenvironment signatures: new diagnostic insights for triple-negative breast cancer
- PMID: 37666492
- PMCID: PMC10552887
- DOI: 10.1002/1878-0261.13515
Assay-agnostic spatial profiling detects tumor microenvironment signatures: new diagnostic insights for triple-negative breast cancer
Abstract
The role of the tumor microenvironment (TME) in immuno-oncology has driven demand for technologies that deliver in situ, or spatial, molecular information. Compartmentalized heterogeneity that traditional methods miss is becoming key to predicting both acquired drug resistance to targeted therapies and patient response to immunotherapy. Here, we describe a novel method for assay-agnostic spatial profiling and demonstrate its ability to detect immune microenvironment signatures in breast cancer patients that are unresolved by the immunohistochemical (IHC) assessment of programmed cell death ligand-1 (PD-L1) on immune cells, which represents the only FDA microenvironment-based companion diagnostic test that has been approved for triple-negative breast cancer (TNBC). Two distinct physiological states were found that are uncorrelated to tumor mutational burden (TMB), microsatellite instability (MSI), PD-L1 expression, and intrinsic cancer subtypes.
Keywords: immuno-oncology; spatial profiling; triple-negative breast cancer; tumor microenvironment.
© 2023 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
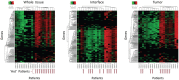
Figures



References
-
- Pitt JM, Marabelle A, Eggermont A, Soria J. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27:1–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

