Microwave synthesis of molybdenene from MoS2
- PMID: 37666941
- PMCID: PMC10716048
- DOI: 10.1038/s41565-023-01484-2
Microwave synthesis of molybdenene from MoS2
Abstract
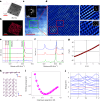
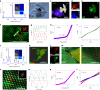
Dirac materials are characterized by the emergence of massless quasiparticles in their low-energy excitation spectrum that obey the Dirac Hamiltonian. Known examples of Dirac materials are topological insulators, d-wave superconductors, graphene, and Weyl and Dirac semimetals, representing a striking range of fundamental properties with potential disruptive applications. However, none of the Dirac materials identified so far shows metallic character. Here, we present evidence for the formation of free-standing molybdenene, a two-dimensional material composed of only Mo atoms. Using MoS2 as a precursor, we induced electric-field-assisted molybdenene growth under microwave irradiation. We observe the formation of millimetre-long whiskers following screw-dislocation growth, consisting of weakly bonded molybdenene sheets, which, upon exfoliation, show metallic character, with an electrical conductivity of ~940 S m-1. Molybdenene when hybridized with two-dimensional h-BN or MoS2, fetch tunable optical and electronic properties. As a proof of principle, we also demonstrate applications of molybdenene as a surface-enhanced Raman spectroscopy platform for molecular sensing, as a substrate for electron imaging and as a scanning probe microscope cantilever.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Novoselov, K. S. et al. Electric field in atomically thin carbon films. Science306, 666–669 (2004). - PubMed
-
- Feng, B. et al. Experimental realization of two-dimensional boron sheets. Nat. Chem.8, 563–568 (2016). - PubMed
-
- Ranjan, P. et al. Freestanding borophene and its hybrids. Adv. Mater.31, 900353 (2019). - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

