High proinsulin:C-peptide ratio identifies individuals with stage 2 type 1 diabetes at high risk for progression to clinical diagnosis and responses to teplizumab treatment
- PMID: 37667106
- PMCID: PMC10914155
- DOI: 10.1007/s00125-023-06003-5
High proinsulin:C-peptide ratio identifies individuals with stage 2 type 1 diabetes at high risk for progression to clinical diagnosis and responses to teplizumab treatment
Abstract
Aims/hypothesis: Tractable precision biomarkers to identify immunotherapy responders are lacking in type 1 diabetes. We hypothesised that proinsulin:C-peptide (PI:C) ratios, a readout of beta cell stress, could provide insight into type 1 diabetes progression and responses to immunotherapy.
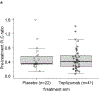
Methods: In this post hoc analysis, proinsulin and C-peptide levels were determined in baseline serum samples from 63 participants with stage 2 type 1 diabetes in the longitudinal TrialNet Teplizumab Prevention Study (n=41 in the teplizumab arm; n=22 in the placebo arm). In addition, previously tested demographic, C-peptide, glucose and proinsulin data were used for the new data analyses. The ratio of intact (unprocessed) proinsulin to C-peptide was analysed and relationships with progression to stage 3 diabetes were investigated.
Results: Elevated baseline PI:C was strongly associated with more rapid progression of diabetes in both the placebo and teplizumab treatment groups, but teplizumab abrogated the impact of high pre-treatment PI:C on type 1 diabetes progression. Differential responses of drug treatment in those with high vs low PI:C ratios were independent of treatment effects of teplizumab on the PI:C ratio or on relevant immune cells.
Conclusions/interpretation: High pre-treatment PI:C identified individuals with stage 2 type 1 diabetes who were exhibiting rapid progression to stage 3 disease and who displayed benefit from teplizumab treatment. These data suggest that readouts of active disease, such as PI:C ratio, could serve to identify optimal candidates or timing for type 1 diabetes disease-modifying therapies.
Keywords: Beta cell; Beta cell dysfunction; Beta cell stress; Biomarker; Precision medicine; Proinsulin; Teplizumab; Type 1 diabetes; Type 1 diabetes prevention.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Conflict of interest: EKS has received compensation for educational lectures on diabetes screening from Medscape and Health Matters CME. KCH has consulted for Provention Bio and is a co-inventor on a patent application for use of teplizumab for delay of Stage 3 type 1 diabetes but without financial remuneration.
Figures





References
-
- Brozzi F, Nardelli TR, Lopes M, Millard I, Barthson J, Igoillo-Esteve M, Grieco FA, Villate O, Oliveira JM, Casimir M, Bugliani M, Engin F, Hotamisligil GS, Marchetti P, Eizirik DL: Cytokines induce endoplasmic reticulum stress in human, rat and mouse beta cells via different mechanisms. Diabetologia 2015;58:2307–2316 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK129523/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- R01 DK121843/DK/NIDDK NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- U01 DK085476/DK/NIDDK NIH HHS/United States
- R01 DK133881/DK/NIDDK NIH HHS/United States
- R01 DK121929/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK106993/DK/NIDDK NIH HHS/United States
- R01 DK057846/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- U01 DK127382/DK/NIDDK NIH HHS/United States
- P30 DK097512/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- UC4 DK097835/DK/NIDDK NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

