Enhanced detection rate of Mycoplasma genitalium in urine overtime by transcription-mediated amplification in comparison to real-time PCR
- PMID: 37667184
- PMCID: PMC10476297
- DOI: 10.1186/s12879-023-08499-z
Enhanced detection rate of Mycoplasma genitalium in urine overtime by transcription-mediated amplification in comparison to real-time PCR
Abstract
Background: Diagnosis of infected individuals with Mycoplasma genitalium (MG) is often performed by real-time PCR or transcription-mediated amplification (TMA). A limitation of the MG-TMA assay is the relatively short time span of 24 h in which the collected urine is required to be transferred into a Urine Specimen Transport Tube, according to the manufacturer's guidelines. If not transferred within 24 h, the manufacturer's claimed sensitivity cannot be guaranteed anymore, and samples may instead be tested with an in-house validated real-time PCR, despite its recognized lower sensitivity. This study aimed to validate an exception to the sample transport and storage conditions of the MG-TMA assay as set by the manufacturer, being the prolongation of the acceptable testing time limit of 24 h.
Methods: From June to December 2022, first-void urines were collected from clients attending the clinic for sexual health in Amsterdam, the Netherlands. Urine samples that tested positive for MG by TMA assay at the day of collection were concomitantly stored at room (18-24 °C) and refrigerator temperature (4-8 °C) for 15 days. The stored urine samples were tested with both an in-house validated real-time PCR and MG-TMA assay after transfer of the original urine samples to the respective test tubes at 3, 7, 12 and 15 days post collection.
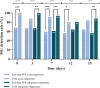
Results: In total, 47 MG-positive urine samples were collected, stored and tested for MG by real-time PCR and TMA assays. After storage at room temperature, the MG-detection rate by TMA was significantly higher compared to real-time PCR, at days 0 (p ≤ 0.001), 7 (p ≤ 0.001) and 12 (p < 0.05). After storage at refrigerator temperature, the MG-detection rate determined by TMA assay was significantly enhanced in comparison with real-time PCR at days 3 (p < 0.01), 7 (p ≤ 0.001) and 15 (p < 0.01).
Conclusions: This validation study showed that the MG-TMA assay has a superior detection rate in urine compared to real-time PCR, up to 15 days post sample collection and irrespective of storage temperature. Accepting urines older than 24 h to be tested by TMA will improve clinical diagnosis of MG infections.
Keywords: Antimicrobial resistance; Molecular diagnostic techniques; Mycoplasma genitalium; Nucleic acid amplification test; Sexually transmitted infection; Transcription-mediated amplification.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Gnanadurai R, Fifer H. Mycoplasma genitalium: a review. (1465–2080 (Electronic)). - PubMed
-
- Jensen JS, Björnelius E, Dohn B, Lidbrink P. Use of TaqMan 5’ nuclease real-time PCR for quantitative detection of Mycoplasma genitalium DNA in males with and without urethritis who were attendees at a sexually transmitted disease clinic. J Clin Microbiol. 2004;42(2):683–92. doi: 10.1128/JCM.42.2.683-692.2004. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

