Regulation of anoikis by extrinsic death receptor pathways
- PMID: 37667281
- PMCID: PMC10478316
- DOI: 10.1186/s12964-023-01247-5
Regulation of anoikis by extrinsic death receptor pathways
Abstract
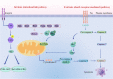
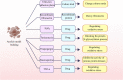
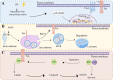
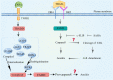
Metastatic cancer cells can develop anoikis resistance in the absence of substrate attachment and survive to fight tumors. Anoikis is mediated by endogenous mitochondria-dependent and exogenous death receptor pathways, and studies have shown that caspase-8-dependent external pathways appear to be more important than the activity of the intrinsic pathways. This paper reviews the regulation of anoikis by external pathways mediated by death receptors. Different death receptors bind to different ligands to activate downstream caspases. The possible mechanisms of Fas-associated death domain (FADD) recruitment by Fas and TNF receptor 1 associated-death domain (TRADD) recruitment by tumor necrosis factor receptor 1 (TNFR1), and DR4- and DR5-associated FADD to induce downstream caspase activation and regulate anoikis were reviewed. This review highlights the possible mechanism of the death receptor pathway mediation of anoikis and provides new insights and research directions for studying tumor metastasis mechanisms. Video Abstract.
Keywords: Aanoikis; Cancer metastasis; DR4/DR5; Death receptor pathway; Fas; TNFR1/TNFR2.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

