Evidence for adolescent length growth spurts in bonobos and other primates highlights the importance of scaling laws
- PMID: 37667589
- PMCID: PMC10479963
- DOI: 10.7554/eLife.86635
Evidence for adolescent length growth spurts in bonobos and other primates highlights the importance of scaling laws
Abstract
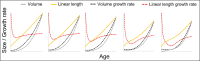
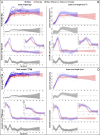
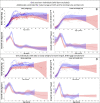
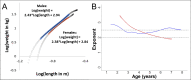
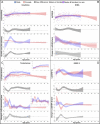
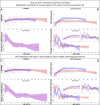
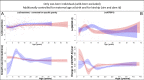
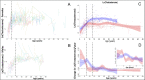
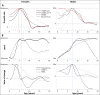
Adolescent growth spurts (GSs) in body length seem to be absent in non-human primates and are considered a distinct human trait. However, this distinction between present and absent length-GSs may reflect a mathematical artefact that makes it arbitrary. We first outline how scaling issues and inappropriate comparisons between length (linear) and weight (volume) growth rates result in misleading interpretations like the absence of length-GSs in non-human primates despite pronounced weight-GSs, or temporal delays between length- and weight-GSs. We then apply a scale-corrected approach to a comprehensive dataset on 258 zoo-housed bonobos that includes weight and length growth as well as several physiological markers related to growth and adolescence. We found pronounced GSs in body weight and length in both sexes. Weight and length growth trajectories corresponded with each other and with patterns of testosterone and insulin-like growth factor-binding protein 3 levels, resembling adolescent GSs in humans. We further re-interpreted published data of non-human primates, which showed that aligned GSs in weight and length exist not only in bonobos. Altogether, our results emphasize the importance of considering scaling laws when interpreting growth curves in general, and further show that pronounced, human-like adolescent length-GSs exist in bonobos and probably also many other non-human primates.
Keywords: Pan paniscus; evolutionary biology; length growth; ontogeny; physiological non-invasive marker; scaling issues; weight growth.
© 2023, Berghaenel, Stevens et al.
Conflict of interest statement
AB, JS, GH, TD, VB No competing interests declared
Figures

Update of
- doi: 10.1101/2023.01.26.525764
- doi: 10.7554/eLife.86635.1
- doi: 10.7554/eLife.86635.2
References
-
- Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophotonics International. 2004;11:36–42.
-
- Alberti C, Chevenne D, Mercat I, Josserand E, Armoogum-Boizeau P, Tichet J, Léger J. Serum concentrations of insulin-like growth factor (IGF)-1 and IGF binding protein-3 (IGFBP-3), IGF-1/IGFBP-3 ratio, and markers of bone turnover: reference values for French children and adolescents and z-score comparability with other references. Clinical Chemistry. 2011;57:1424–1435. doi: 10.1373/clinchem.2011.169466. - DOI - PubMed
-
- Anzà S, Berghänel A, Ostner J, Schülke O. Growth trajectories of wild Assamese macaques (Macaca assamensis) determined from parallel laser photogrammetry. Mammalian Biology. 2022;102:1497–1511. doi: 10.1007/s42991-022-00262-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

