NKRF in Cardiac Fibroblasts Protects against Cardiac Remodeling Post-Myocardial Infarction via Human Antigen R
- PMID: 37667861
- PMCID: PMC10602562
- DOI: 10.1002/advs.202303283
NKRF in Cardiac Fibroblasts Protects against Cardiac Remodeling Post-Myocardial Infarction via Human Antigen R
Abstract
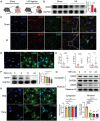
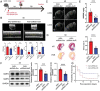
Myocardial infarction (MI) remains the leading cause of death worldwide. Cardiac fibroblasts (CFs) are abundant in the heart and are responsible for cardiac repair post-MI. NF-κB-repressing factor (NKRF) plays a significant role in the transcriptional inhibition of various specific genes. However, the NKRF action mechanism in CFs remains unclear in cardiac repair post-MI. This study investigates the NKRF mechanism in cardiac remodeling and dysfunction post-MI by establishing a CF-specific NKRF-knockout (NKRF-CKO) mouse model. NKRF expression is downregulated in CFs in response to pathological cardiac remodeling in vivo and TNF-α in vitro. NKRF-CKO mice demonstrate worse cardiac function and survival and increased infarct size, heart weight, and MMP2 and MMP9 expression post-MI compared with littermates. NKRF inhibits CF migration and invasion in vitro by downregulating MMP2 and MMP9 expression. Mechanistically, NKRF inhibits human antigen R (HuR) transcription by binding to the classical negative regulatory element within the HuR promoter via an NF-κB-dependent mechanism. This decreases HuR-targeted Mmp2 and Mmp9 mRNA stability. This study suggests that NKRF is a therapeutic target for pathological cardiac remodeling.
Keywords: NF-κB-repressing factor; cardiac fibroblasts; human antigen R; myocardial infarction; transcriptional and post-transcriptional regulation.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- a) Hashimoto H., Olson E. N., Bassel‐Duby R., Nat. Rev. Cardiol. 2018, 15, 585; - PMC - PubMed
- b) Zhou M., Wang H., Zeng X., Yin P., Zhu J., Chen W., Li X., Wang L., Wang L., Liu Y., Liu J., Zhang M., Qi J., Yu S., Afshin A., Gakidou E., Glenn S., Krish V. S., Miller‐Petrie M. K., Mountjoy‐Venning W. C., Mullany E. C., Redford S. B., Liu H., Naghavi M., Hay S. I., Wang L., Murray C. J. L., Liang X., Lancet 2019, 394, 1145. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 81920108003/National Natural Science Foundation of China
- 82241203/National Natural Science Foundation of China
- 82270487/National Natural Science Foundation of China
- 81970373/National Natural Science Foundation of China
- 31770977/National Natural Science Foundation of China
- 82030051/National Natural Science Foundation of China
- 81900321/National Natural Science Foundation of China
- 82270331/National Natural Science Foundation of China
- 82070453/National Natural Science Foundation of China
- 2021YFF0501403/National Key Research and Development Program of China
- 2021ZLGX02/Key R&D Program of Shandong Province
- 2018M630789/Postdoctoral Science Foundation of China and Shandong Province
- 201901009/Postdoctoral Science Foundation of China and Shandong Province
- ZR2020YQ53/Shandong Provincial Natural Science Foundation
- 2021ZDSYS05/Shandong Provincial Natural Science Foundation
- ZR2023JQ030/Shandong Provincial Natural Science Foundation
- Taishan Scholars Program of Shandong Province
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
