First-morning urine osmolality and nocturnal enuresis in children: A single-center prospective cohort study
- PMID: 37668207
- PMCID: PMC10482672
- DOI: 10.4111/icu.20220377
First-morning urine osmolality and nocturnal enuresis in children: A single-center prospective cohort study
Abstract
Purpose: To investigate the treatment outcome of nocturnal enuresis (NE) according to first-morning urine osmolality (Uosm) before treatment.
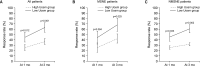
Materials and methods: Ninety-nine children (mean age, 7.2±2.1 y) with NE were enrolled in this retrospective study and divided into two groups according to first-morning Uosm results, that is, into a low Uosm group (<800 mOsm/L; 38 cases, 38.4%) or a high Uosm group (≥800 mOsm/L; 61 cases, 61.6%). Baseline parameters were obtained from frequency volume charts of at least 2 days, uroflowmetry, post-void residual volume, and a questionnaire for the presence of frequency, urgency, and urinary incontinence. Standard urotherapy and pharmacological treatment were administered initially in all cases. Enuresis frequency and response rates were analyzed at around 1 month and 3 months after treatment initiation.
Results: The level of first-morning Uosm was 997.1±119.6 mOsm/L in high Uosm group and 600.9±155.9 mOsm/L in low Uosm group (p<0.001), and first-morning voided volume (p=0.021) and total voided volume (p=0.019) were significantly greater in the low Uosm group. Furthermore, a significantly higher percentage of children in the low Uosm group had a response rate of ≥50% (CR or PR) at 1 month (50.0% vs. 24.6%; p=0.010) and 3 months (63.2% vs. 36.1%; p=0.009).
Conclusions: Treatment response rates are higher for children with NE with a lower first-morning Uosm.
Keywords: Child; Nocturnal enuresis; Osmolar concentration; Urinalysis; Urinary bladder.
© The Korean Urological Association.
Conflict of interest statement
The authors have nothing to disclose.
Figures

Similar articles
-
Pretreatment morning urine osmolality and oral desmopressin lyophilisate treatment outcome in patients with primary monosymptomatic enuresis.Int Urol Nephrol. 2021 Aug;53(8):1529-1534. doi: 10.1007/s11255-021-02843-5. Epub 2021 Mar 27. Int Urol Nephrol. 2021. PMID: 33774753
-
Diagnostic value of functional bladder capacity, urine osmolality, and daytime storage symptoms for severity of nocturnal enuresis.Korean J Urol. 2012 Feb;53(2):114-9. doi: 10.4111/kju.2012.53.2.114. Epub 2012 Feb 20. Korean J Urol. 2012. PMID: 22379591 Free PMC article.
-
Evaluation of nocturnal bladder capacity and nocturnal urine volume in nocturnal enuresis.J Pediatr Urol. 2014 Jun;10(3):559-63. doi: 10.1016/j.jpurol.2013.11.020. Epub 2013 Dec 21. J Pediatr Urol. 2014. PMID: 24388899
-
24-h hydration status: parameters, epidemiology and recommendations.Eur J Clin Nutr. 2003 Dec;57 Suppl 2:S10-8. doi: 10.1038/sj.ejcn.1601896. Eur J Clin Nutr. 2003. PMID: 14681708 Review.
-
Clinical management of nocturnal enuresis.Pediatr Nephrol. 2018 Jul;33(7):1145-1154. doi: 10.1007/s00467-017-3778-1. Epub 2017 Aug 21. Pediatr Nephrol. 2018. PMID: 28828529 Review.
References
-
- Robson WL. Clinical practice. Evaluation and management of enuresis. N Engl J Med. 2009;360:1429–1436. - PubMed
-
- Chung JM, Lee SD, Kang DI, Kwon DD, Kim KS, Kim SY, et al. Korean Enuresis Association. An epidemiologic study of voiding and bowel habits in Korean children: a nationwide multicenter study. Urology. 2010;76:215–219. - PubMed
-
- Walle JV, Rittig S, Bauer S, Eggert P, Marschall-Kehrel D, Tekgul S American Academy of Pediatrics; European Society for Paediatric Urology; European Society for Paediatric Nephrology; International Children’s Continence Society. Practical consensus guidelines for the management of enuresis. Eur J Pediatr. 2012;171:971–983. Erratum in: Eur J Pediatr 2013;172:285. - PMC - PubMed
-
- Tullus K, Bergström R, Fosdal I, Winnergård I, Hjälmås K. Efficacy and safety during long-term treatment of primary monosymptomatic nocturnal enuresis with desmopressin. Swedish Enuresis Trial Group. Acta Paediatr. 1999;88:1274–1278. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

