Multiplexity of human brain oscillations as a personal brain signature
- PMID: 37668332
- PMCID: PMC10619372
- DOI: 10.1002/hbm.26466
Multiplexity of human brain oscillations as a personal brain signature
Abstract
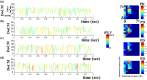
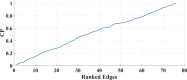
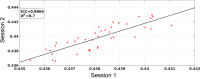
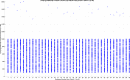
Human individuality is likely underpinned by the constitution of functional brain networks that ensure consistency of each person's cognitive and behavioral profile. These functional networks should, in principle, be detectable by noninvasive neurophysiology. We use a method that enables the detection of dominant frequencies of the interaction between every pair of brain areas at every temporal segment of the recording period, the dominant coupling modes (DoCM). We apply this method to brain oscillations, measured with magnetoencephalography (MEG) at rest in two independent datasets, and show that the spatiotemporal evolution of DoCMs constitutes an individualized brain fingerprint. Based on this successful fingerprinting we suggest that DoCMs are important targets for the investigation of neural correlates of individual psychological parameters and can provide mechanistic insight into the underlying neurophysiological processes, as well as their disturbance in brain diseases.
Keywords: MEG; Multiplexity; chronnectomics; dominant coupling modes; individual fingerprint; resting-state; signal processing; time-varying network analysis.
© 2023 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they do not have any conflict of interest.
Figures



Similar articles
-
The brain's resting-state activity is shaped by synchronized cross-frequency coupling of neural oscillations.Neuroimage. 2015 May 1;111:26-35. doi: 10.1016/j.neuroimage.2015.01.054. Epub 2015 Feb 11. Neuroimage. 2015. PMID: 25680519 Free PMC article.
-
MEG source imaging method using fast L1 minimum-norm and its applications to signals with brain noise and human resting-state source amplitude images.Neuroimage. 2014 Jan 1;84:585-604. doi: 10.1016/j.neuroimage.2013.09.022. Epub 2013 Sep 19. Neuroimage. 2014. PMID: 24055704 Free PMC article.
-
Reconfiguration of dominant coupling modes in mild traumatic brain injury mediated by δ-band activity: A resting state MEG study.Neuroscience. 2017 Jul 25;356:275-286. doi: 10.1016/j.neuroscience.2017.05.032. Epub 2017 May 31. Neuroscience. 2017. PMID: 28576727
-
Alzheimer's disease: The state of the art in resting-state magnetoencephalography.Clin Neurophysiol. 2017 Aug;128(8):1426-1437. doi: 10.1016/j.clinph.2017.05.012. Epub 2017 May 21. Clin Neurophysiol. 2017. PMID: 28622527 Review.
-
From bench to bedside: Overview of magnetoencephalography in basic principle, signal processing, source localization and clinical applications.Neuroimage Clin. 2024;42:103608. doi: 10.1016/j.nicl.2024.103608. Epub 2024 Apr 20. Neuroimage Clin. 2024. PMID: 38653131 Free PMC article. Review.
Cited by
-
Demonstrating equivalence across magnetoencephalography scanner platforms using neural fingerprinting.Imaging Neurosci (Camb). 2025 May 21;3:IMAG.a.10. doi: 10.1162/IMAG.a.10. eCollection 2025. Imaging Neurosci (Camb). 2025. PMID: 40800955 Free PMC article.
-
Major individual and regional variations in unit entrainment by oscillations of different frequencies.Sci Rep. 2025 Jan 13;15(1):1772. doi: 10.1038/s41598-025-85914-2. Sci Rep. 2025. PMID: 39800772 Free PMC article.
References
-
- Amunts, K. , Malikovic, A. , Mohlberg, H. , Schormann, T. , & Zilles, K. (2000). Brodmann's areas 17 and 18 brought into stereotaxic space‐where and how variable? NeuroImage, 11, 66–84. - PubMed
-
- Aru, J. , Aru, J. , Priesemann, V. , Wibral, M. , Lana, L. , Pipa, G. , Singer, W. , & Vicente, R. (2015). Untangling cross‐frequency coupling in neuroscience. Current Opinion in Neurobiology, 31, 51–61. - PubMed
-
- Barch, D. M. , Burgess, G. C. , Harms, M. P. , Petersen, S. E. , Schlaggar, B. L. , Corbetta, M. , Glasser, M. F. , Curtiss, S. , Dixit, S. , Feldt, C. , Nolan, D. , Bryant, E. , Hartley, T. , Footer, O. , Bjork, J. M. , Poldrack, R. , Smith, S. , Johansen‐Berg, H. , Snyder, A. Z. , … WU‐Minn HCP Consortium . (2013). Function in the human connectome: Task‐fMRI and individual differences in behavior. NeuroImage, 80, 169–189. - PMC - PubMed
-
- Başar, E. , Schmiedt‐Fehr, C. , Mathes, B. , Femir, B. , Emek‐Savaş, D. D. , Tülay, E. , Tan, D. , Düzgün, A. , Güntekin, B. , Özerdem, A. , Yener, G. , & Başar‐Eroğlu, C. (2016). What does the broken brain say to the neuroscientist? Oscillations and connectivity in schizophrenia, Alzheimer's disease, and bipolar disorder. International Journal of Psychophysiology, 103, 135–148. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

