Blood Pressure Management After Endovascular Therapy for Acute Ischemic Stroke: The BEST-II Randomized Clinical Trial
- PMID: 37668620
- PMCID: PMC10481231
- DOI: 10.1001/jama.2023.14330
Blood Pressure Management After Endovascular Therapy for Acute Ischemic Stroke: The BEST-II Randomized Clinical Trial
Abstract
Importance: The effects of moderate systolic blood pressure (SBP) lowering after successful recanalization with endovascular therapy for acute ischemic stroke are uncertain.
Objective: To determine the futility of lower SBP targets after endovascular therapy (<140 mm Hg or 160 mm Hg) compared with a higher target (≤180 mm Hg).
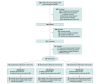
Design, setting, and participants: Randomized, open-label, blinded end point, phase 2, futility clinical trial that enrolled 120 patients with acute ischemic stroke who had undergone successful endovascular therapy at 3 US comprehensive stroke centers from January 2020 to March 2022 (final follow-up, June 2022).
Intervention: After undergoing endovascular therapy, participants were randomized to 1 of 3 SBP targets: 40 to less than 140 mm Hg, 40 to less than 160 mm Hg, and 40 to 180 mm Hg or less (guideline recommended) group, initiated within 60 minutes of recanalization and maintained for 24 hours.
Main outcomes and measures: Prespecified multiple primary outcomes for the primary futility analysis were follow-up infarct volume measured at 36 (±12) hours and utility-weighted modified Rankin Scale (mRS) score (range, 0 [worst] to 1 [best]) at 90 (±14) days. Linear regression models were used to test the harm-futility boundaries of a 10-mL increase (slope of 0.5) in the follow-up infarct volume or a 0.10 decrease (slope of -0.005) in the utility-weighted mRS score with each 20-mm Hg SBP target reduction after endovascular therapy (1-sided α = .05). Additional prespecified futility criterion was a less than 25% predicted probability of success for a future 2-group, superiority trial comparing SBP targets of the low- and mid-thresholds with the high-threshold (maximum sample size, 1500 with respect to the utility-weighted mRS score outcome).
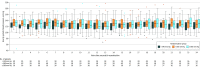
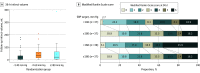
Results: Among 120 patients randomized (mean [SD] age, 69.6 [14.5] years; 69 females [58%]), 113 (94.2%) completed the trial. The mean follow-up infarct volume was 32.4 mL (95% CI, 18.0 to 46.7 mL) for the less than 140-mm Hg group, 50.7 mL (95% CI, 33.7 to 67.7 mL), for the less than 160-mm Hg group, and 46.4 mL (95% CI, 24.5 to 68.2 mL) for the 180-mm Hg or less group. The mean utility-weighted mRS score was 0.51 (95% CI, 0.38 to 0.63) for the less than 140-mm Hg group, 0.47 (95% CI, 0.35 to 0.60) for the less than 160-mm Hg group, and 0.58 (95% CI, 0.46 to 0.71) for the high-target group. The slope of the follow-up infarct volume for each mm Hg decrease in the SBP target, adjusted for the baseline Alberta Stroke Program Early CT score, was -0.29 (95% CI, -0.81 to ∞; futility P = .99). The slope of the utility-weighted mRS score for each mm Hg decrease in the SBP target after endovascular therapy, adjusted for baseline utility-weighted mRS score, was -0.0019 (95% CI, -∞ to 0.0017; futility P = .93). Comparing the high-target SBP group with the lower-target groups, the predicted probability of success for a future trial was 25% for the less than 140-mm Hg group and 14% for the 160-mm Hg group.
Conclusions and relevance: Among patients with acute ischemic stroke, lower SBP targets less than either 140 mm Hg or 160 mm Hg after successful endovascular therapy did not meet prespecified criteria for futility compared with an SBP target of 180 mm Hg or less. However, the findings suggested a low probability of benefit from lower SBP targets after endovascular therapy if tested in a future larger trial.
Trial registration: ClinicalTrials.gov Identifier: NCT04116112.
Conflict of interest statement
Figures
Comment in
-
Blood Pressure Management After Successful Thrombectomy.JAMA. 2023 Sep 5;330(9):811-812. doi: 10.1001/jama.2023.14588. JAMA. 2023. PMID: 37668632 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

