Testing the Real-World Accuracy of the Dexcom G6 Pro CGM During the Insulin-Only Bionic Pancreas Pivotal Trial
- PMID: 37668666
- PMCID: PMC10771867
- DOI: 10.1089/dia.2023.0287
Testing the Real-World Accuracy of the Dexcom G6 Pro CGM During the Insulin-Only Bionic Pancreas Pivotal Trial
Abstract
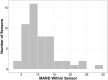
Continuous glucose monitors (CGMs) have transformed the way people with type 1 diabetes can self-monitor glucose levels. Past studies have evaluated the accuracy of CGMs in clinic-based studies, but few have analyzed their accuracy in real-world settings. The Insulin-Only Bionic Pancreas Trial provided the opportunity to assess real-world accuracy of the blinded Dexcom G6 Pro sensor over the first 48-60 h of wear using a blood glucose meter (BGM) as a comparator for 1073 CGM-BGM pairs across 53 participants. The mean absolute relative difference (MARD) was 11.0% over a median period of 50 h (range 47-79 h). The MARD was 13.6% in the first 12 h, 10.5% in hours 12-24, and 10.1% after the first 24 h. These results are comparable with accuracy shown previously with laboratory-based measurements and provide real-world evidence of Dexcom G6 Pro accuracy, which improved after the first 12 h and then remained stable thereafter. Clinical Trial Registry: clinicaltrials.gov; NCT04200313.
Keywords: Accuracy; Blood glucose; Continuous glucose monitors; Dexcom; Type 1 diabetes.
Conflict of interest statement
M.C.M. has no personal financial disclosures but reports that his employer has received grant support from Beta Bionics, Dexcom, and Tandem Diabetes Care. P.C.'s employer has received consulting payments on his behalf from vTv Therapeutics, Beta Bionics, Dexcom, and Diasome. E.R.D. has issued patents and pending patents on aspects of the bionic pancreas, and is an employee, the executive chair of the board of directors, and shareholder of Beta Bionics.
S.J.R. has issued patents and pending patents on aspects of the bionic pancreas that are assigned to Massachusetts General Hospital and licensed to Beta Bionics; has received honoraria and/or travel expenses for lectures from Novo Nordisk, Roche, and Ascensia; serves on the scientific advisory boards of Unomedical; served on scientific advisory board and had stock in Companion Medical that was bought out by Medtronic; has received consulting fees from Beta Bionics, Novo Nordisk, Senseonics, and Flexion Therapeutics; has received grant support from Zealand Pharma, Novo Nordisk, and Beta Bionics; and has received in-kind support in the form of technical support and/or donation of materials from Zealand Pharma, Ascencia, Senseonics, Adocia, and Tandem Diabetes; and is an employee, the chief medical officer, and a shareholder of Beta Bionics.
K.J.R. has no personal financial disclosures but reports that his employer has received grant support from Beta Bionics, Dexcom, and Tandem Diabetes Care. R.W.B. reports no personal financial disclosures but reports that his institution has received funding on his behalf as follows: grant funding and study supplies from Tandem Diabetes Care, Beta Bionics, and Dexcom; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome.
Figures

References
-
- Jafri RZ, Balliro CA, El-Khatib F, et al. . A three-way accuracy comparison of the Dexcom g5, Abbott Freestyle Libre Pro, and senseonics eversense continuous glucose monitoring devices in a home-use study of subjects with type 1 diabetes. Diabetes Technol Ther 2020;22(11):846–852. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

