Risk-stratified faecal immunochemical testing (FIT) for urgent colonoscopy in Lynch syndrome during the COVID-19 pandemic
- PMID: 37668669
- PMCID: PMC10478750
- DOI: 10.1093/bjsopen/zrad079
Risk-stratified faecal immunochemical testing (FIT) for urgent colonoscopy in Lynch syndrome during the COVID-19 pandemic
Erratum in
-
Erratum to: Risk-stratified faecal immunochemical testing (FIT) for urgent colonoscopy in Lynch syndrome during the COVID-19 pandemic.BJS Open. 2023 Nov 1;7(6):zrad153. doi: 10.1093/bjsopen/zrad153. BJS Open. 2023. PMID: 38029326 Free PMC article. No abstract available.
Abstract
Background: Lynch syndrome is a hereditary cancer disease resulting in an increased risk of colorectal cancer. Herein, findings are reported from an emergency clinical service implemented during the COVID-19 pandemic utilizing faecal immunochemical testing ('FIT') in Lynch syndrome patients to prioritize colonoscopy while endoscopy services were limited.
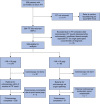
Methods: An emergency service protocol was designed to improve colonoscopic surveillance access throughout the COVID-19 pandemic in England for people with Lynch syndrome when services were extremely restricted (1 March 2020 to 31 March 2021) and promoted by the English National Health Service. Requests for faecal immunochemical testing from participating centres were sent to the National Health Service Bowel Cancer Screening South of England Hub and a faecal immunochemical testing kit, faecal immunochemical testing instructions, paper-based survey, and pre-paid return envelope were sent to patients. Reports with faecal haemoglobin results were returned electronically for clinical action. Risk stratification for colonoscopy was as follows: faecal haemoglobin less than 10 µg of haemoglobin/g of faeces (µg/g)-scheduled within 6-12 weeks; and faecal haemoglobin greater than or equal to 10 µg/g-triaged via an urgent suspected cancer clinical pathway. Primary outcomes of interest included the identification of highest-risk Lynch syndrome patients and determining the impact of faecal immunochemical testing in risk-stratified colonoscopic surveillance.
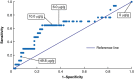
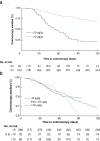
Results: Fifteen centres participated from June 2020 to March 2021. Uptake was 68.8 per cent amongst 558 patients invited. For 339 eligible participants analysed, 279 (82.3 per cent) had faecal haemoglobin less than 10 µg/g and 60 (17.7 per cent) had faecal haemoglobin greater than or equal to 10 µg/g. In the latter group, the diagnostic accuracy of faecal immunochemical testing was 65.9 per cent and escalation to colonoscopy was facilitated (median 49 versus 122 days, χ2 = 0.0003, P < 0.001).
Conclusion: Faecal immunochemical testing demonstrated clinical value for Lynch syndrome patients requiring colorectal cancer surveillance during the pandemic in this descriptive report of an emergency COVID-19 response service. Further longitudinal investigation on faecal immunochemical testing efficacy in Lynch syndrome is warranted and will be examined under the 'FIT for Lynch' study (ISRCTN15740250).
© The Author(s) 2023. Published by Oxford University Press on behalf of BJS Society Ltd.
Figures
References
-
- Monahan KJ, Bradshaw N, Dolwani S, Desouza B, Dunlop MG, East JE et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG). Gut 2020;69:411–444 - PMC - PubMed
-
- British Society of Gastroenterology . GI Endoscopy Activity and COVID-19: Next Steps. https://www.bsg.org.uk/covid-19-advice/gi-endoscopy-activity-and-covid-1... (accessed 2 January 2023)
-
- Rutter MD, Brookes M, Lee TJ, Rogers P, Sharp L. Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a national endoscopy database analysis. Gut 2021;70:537–543 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

