Risk factors for thromboembolic events in patients with paroxysmal nocturnal hemoglobinuria (PNH): a nested case-control study in the International PNH Registry
- PMID: 37668788
- PMCID: PMC10567964
- DOI: 10.1007/s00277-023-05402-3
Risk factors for thromboembolic events in patients with paroxysmal nocturnal hemoglobinuria (PNH): a nested case-control study in the International PNH Registry
Abstract
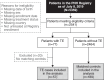
The objective of this analysis was to identify risk factors for thromboembolic events (TE) in patients with paroxysmal nocturnal hemoglobinuria (PNH) who were not treated with C5 inhibitors. Patients with PNH and a history of ≥ 1 TE at enrollment in the International PNH Registry (NCT01374360; registration date, January 2011) were each matched with up to 5 patients without TE. Multivariable analysis was performed with the following variables: percentage glycosylphosphatidylinositol (GPI)-negative cells, high disease activity (HDA), non-TE major adverse vascular event history, and recent anticoagulation. Of 2541 eligible patients, 57 with TE and 189 matched controls were analyzed. Multivariable analysis (odds ratio [95% CI]) identified the following factors as being associated with increased thrombotic risk: patients with no history of TE (with recent anticoagulation, 9.30 [1.20-72.27]), patients with history of TE (with recent anticoagulation, 8.91 [0.86-92.62]; without recent anticoagulation, 5.33 [0.26-109.57]), patients with ≥ 30% GPI-negative granulocytes (≥ 30% to < 50%, 4.94 [0.54-45.32]; ≥ 50%, 1.97 [0.45-8.55]), or patients with lactate dehydrogenase (LDH) ratio ≥ 1.5 × upper limit of normal (ULN) plus ≥ 2 HDA criteria (2-3 criteria, 3.18 [0.44-23.20]; ≥ 4 criteria, 3.60 [0.38-33.95]). History of TE, ≥ 30% GPI-negative granulocytes, and LDH ratio ≥ 1.5 × ULN with ≥ 2 HDA criteria are TE risk factors for patients with PNH. These findings will aid physicians by providing important clinical and laboratory risk factors that can be used to identify and manage patients with PNH who are at risk of developing TE.
Keywords: Cohort study; Multivariable analysis; Paroxysmal nocturnal hemoglobinuria; Risk factors; Thromboembolism.
© 2024. The Author(s).
Conflict of interest statement
Declaration. Ethics approval: The institutional review boards (or equivalent) of participating centers approved the Registry. Consent to participate/Consent to publish: Written informed consent was obtained from all individual participants who were included in the International PNH Registry. Competing interests: B Höchsmann has received honoraria, consulting fees, and research support (to University of Ulm) from Alexion, AstraZeneca Rare Disease; Novartis; Ra Pharma; Roche; Sobi; and Apellis. R Peffault de Latour has received honoraria, consulting fees, and research support from Alexion, AstraZeneca Rare Disease; Pfizer; and Novartis; and has received research support from Amgen. A Hill has received honoraria and/or consulting fees from Akari Therapeutics; Alexion, AstraZeneca Rare Disease; Apellis; Bioverativ; Novartis; Ra Pharma; Regeneron; and Roche; and is currently employed by Alexion, AstraZeneca Rare Disease. A Röth has received honoraria from Alexion, AstraZeneca Rare Disease; Roche Pharma; Novartis; Sanofi; Bioverativ; BioCryst; Sobi; Apellis; and Kira. T Devos has received consultancy fees from Alexion, AstraZeneca Rare Disease; Gilead; Novartis; and Janssen; and has participated in advisory boards for Novartis, Gilead, and Janssen. CJ Patriquin has received an unrestricted educational grant from Alexion, AstraZeneca Rare Disease. He has participated in advisory boards with Alexion, AstraZeneca Rare Disease; Apellis; Sanofi; and Biocryst. He is/has been site investigator for trials with Alexion, AstraZeneca Rare Disease; Apellis; Ra Pharma; and Sanofi. W–C Chou has nothing to declare. D Jain is employed by Alexion, AstraZeneca Rare Disease. K Zu and C Wu were employed by Alexion, AstraZeneca Rare Disease, at the time of study. JW Lee has received honoraria, consulting fees, and research support from Alexion, AstraZeneca Rare Disease.
Figures



Similar articles
-
Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review.Int J Mol Sci. 2025 Mar 11;26(6):2504. doi: 10.3390/ijms26062504. Int J Mol Sci. 2025. PMID: 40141144 Free PMC article. Review.
-
Relationship of paroxysmal nocturnal hemoglobinuria (PNH) granulocyte clone size to disease burden and risk of major vascular events in untreated patients: results from the International PNH Registry.Ann Hematol. 2023 Jul;102(7):1637-1644. doi: 10.1007/s00277-023-05269-4. Epub 2023 May 18. Ann Hematol. 2023. PMID: 37199789 Free PMC article. Review.
-
Baseline clinical characteristics and disease burden in patients with paroxysmal nocturnal hemoglobinuria (PNH): updated analysis from the International PNH Registry.Ann Hematol. 2020 Jul;99(7):1505-1514. doi: 10.1007/s00277-020-04052-z. Epub 2020 May 10. Ann Hematol. 2020. PMID: 32390114 Free PMC article.
-
Impact of Lactate Dehydrogenase and Hemoglobin Levels on Clinical Outcomes in Patients With Paroxysmal Nocturnal Hemoglobinuria: Results From the National Korean PNH Registry.J Korean Med Sci. 2024 Mar 4;39(8):e81. doi: 10.3346/jkms.2024.39.e81. J Korean Med Sci. 2024. PMID: 38442722 Free PMC article.
-
Effect of eculizumab treatment in patients with paroxysmal nocturnal hemoglobinuria with or without high disease activity: Real-world findings from the International Paroxysmal Nocturnal Hemoglobinuria Registry.Eur J Haematol. 2022 Sep;109(3):197-204. doi: 10.1111/ejh.13773. Epub 2022 Jun 21. Eur J Haematol. 2022. PMID: 35390189
Cited by
-
Management of paroxysmal nocturnal hemoglobinuria with low-level hemolysis in pregnancy- a report of two cases.Ann Hematol. 2025 Feb;104(2):1249-1253. doi: 10.1007/s00277-024-06086-z. Epub 2024 Nov 13. Ann Hematol. 2025. PMID: 39537992 Free PMC article.
-
Prevention and Management of Thromboembolism in Patients with Paroxysmal Nocturnal Hemoglobinuria in Asia: A Narrative Review.Int J Mol Sci. 2025 Mar 11;26(6):2504. doi: 10.3390/ijms26062504. Int J Mol Sci. 2025. PMID: 40141144 Free PMC article. Review.
-
Patient-Reported Meaningful Change in Symptoms and Impacts of Paroxysmal Nocturnal Hemoglobinuria (PNH) in Three Phase III Clinical Trials of Iptacopan.Patient. 2025 Jul 22. doi: 10.1007/s40271-025-00755-5. Online ahead of print. Patient. 2025. PMID: 40694294
-
[Guidelines for the diagnosis and management of paroxysmal nocturnal hemoglobinuria (2024)].Zhonghua Xue Ye Xue Za Zhi. 2024 Aug 14;45(8):727-737. doi: 10.3760/cma.j.cn121090-20240624-00232. Zhonghua Xue Ye Xue Za Zhi. 2024. PMID: 39307719 Free PMC article. Chinese.
-
Paroxysmal Nocturnal Hemoglobinuria: Current Management, Unmet Needs, and Recommendations.J Blood Med. 2023 Dec 6;14:613-628. doi: 10.2147/JBM.S431493. eCollection 2023. J Blood Med. 2023. PMID: 38084255 Free PMC article. Review.
References
-
- Patriquin CJ, Kiss T, Caplan S, Chin-Yee I, Grewal K, Grossman J, Larratt L, Marceau D, Nevill T, Sutherland DR, Wells RA, Leber B (2019) How we treat paroxysmal nocturnal hemoglobinuria: a consensus statement of the Canadian PNH Network and review of the national registry. Eur J Haematol 102(1):36–52. 10.1111/ejh.13176 - PubMed
-
- Devos T, Meers S, Boeckx N, Gothot A, Deeren D, Chatelain B, Chatelain C, Devalet B (2018) Diagnosis and management of PNH: Review and recommendations from a Belgian expert panel. Eur J Haematol 101(6):737–749. 10.1111/ejh.13166 - PubMed
-
- Parker C, Omine M, Richards S, Nishimura J, Bessler M, Ware R, Hillmen P, Luzzatto L, Young N, Kinoshita T, Rosse W, Socie G, International P. N. H. Interest Group (2005) Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood 106(12):3699–3709. 10.1182/blood-2005-04-1717 - PMC - PubMed
-
- Socié G, Schrezenmeier H, Muus P, Lisukov I, Röth A, Kulasekararaj A, Lee JW, Araten D, Hill A, Brodsky R, Urbano-Ispizua A, Szer J, Wilson A, Hillmen P, on behalf of the PNH Registry (2016) Changing prognosis in paroxysmal nocturnal haemoglobinuria disease subcategories: an analysis of the International PNH Registry. Intern Med J 46(9):1044–1053. 10.1111/imj.13160 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

